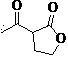
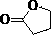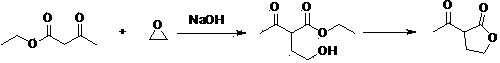Green synthesis process of alpha-acetyl-gamma-butyrolactone
A technology of butyrolactone and acetyl, applied in the field of chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0026] Example: Synthesis of α-acetyl-γ-butyrolactone
[0027] Weigh 108 g γ-butyrolactone and 188 g ethyl acetate and mix well. Weigh 40g of calcium oxide, grind it evenly, and add it into the reaction flask. 100g of mixed esters was quickly added to the reaction flask, the temperature was controlled at 77°C, and the remaining mixed esters were slowly added dropwise. Insulate at 77°C for 20 hours, monitor the completion of the reaction by gas phase monitoring, evaporate the solvent, add 52 g of 18% hydrochloric acid, stir at 82°C for 1 hour, extract with ethyl acetate, concentrate to obtain a red liquid, and rectify to obtain a colorless α-acetyl-γ- Butyrolactone 104.48 g, yield 65%. 1 H NMR (CDCl 3 ):d4.40-4.29 (m, 2H), 3.80-3.76 (m, 1H), 2.77-2.74 (m, 1H), 2.43 (s, 3H), 2.36-2.33 (m, 1H), MS: m / e(129, M+1), elemental analysis C 6 h 8 o 3 , Calcd: C 56.24, H 6.29. Experimental value: C56.21, H6.28.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com