Biphenol compound with anti-tumor activity and pharmaceutical use of compound
A technology for biphenol and antitumor drugs, which is applied in the field of biquinol compounds with antitumor activity and pharmaceutical applications, and can solve the problems of unreported antitumor activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
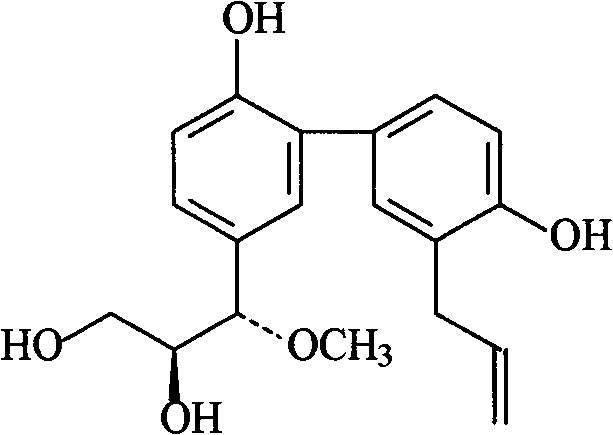
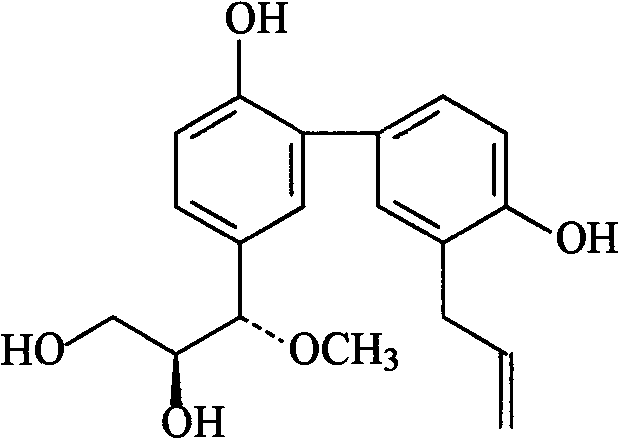
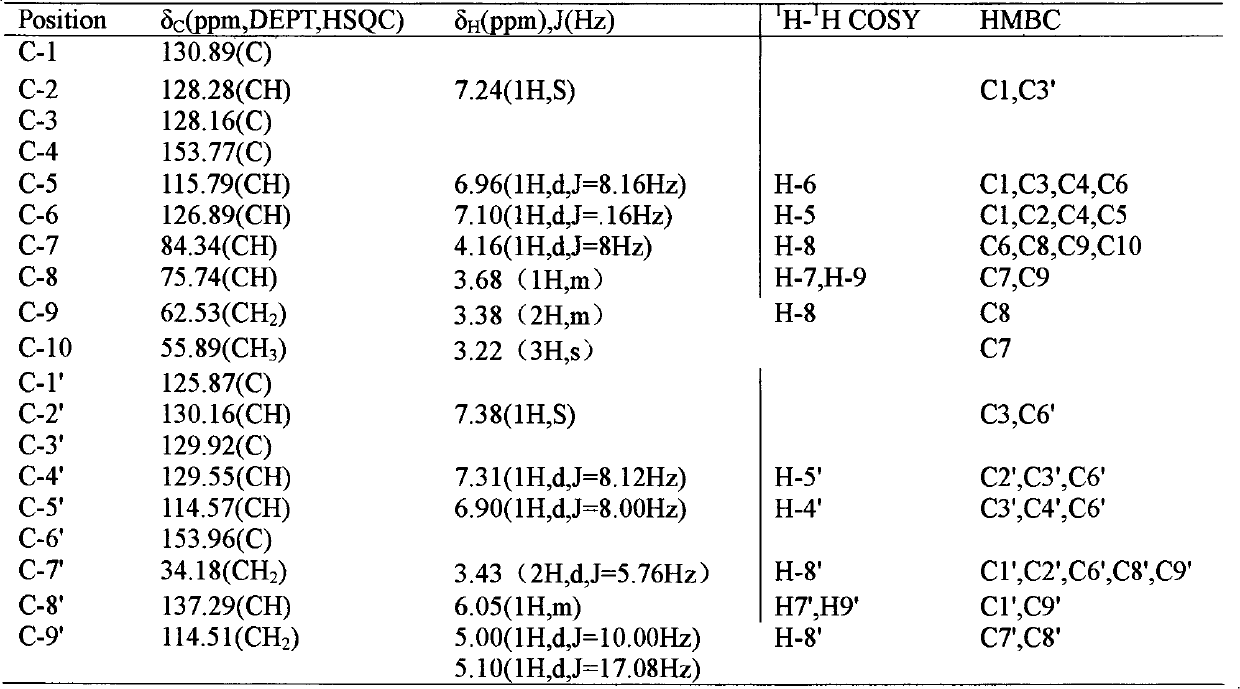
[0030] Embodiment 1: the preparation of formula I compound
[0031] 1. Take 100 kg of Magnolia officinalis medicinal material, crush it into a coarse powder, add 70% ethanol to extract, the amount of ethanol is 8 times of the weight of the medicinal material, each extraction time is 2 hours, and the number of extractions is 3 times.
[0032] 2. Filtrate the extract, combine the filtrates, and concentrate under reduced pressure at 60°C to form an extract.
[0033] 3. Add 10 times the amount of 5% sodium hydroxide solution to the extract, stir to dissolve, let it stand until the solution is clear, filter, then add hydrochloric acid to adjust the pH value to 2-3, stand overnight, filter, and get acid precipitate .
[0034] 3. The acid precipitate was washed with deionized water until neutral, dried and pulverized to obtain the precipitate.
[0035] 4. Take the precipitate, add dichloromethane to reflux and extract, the amount of solvent used is 5 times the weight of the precipi...
Embodiment 2
[0043] Embodiment 2: MTT reduction method detection formula I antitumor activity experiment
[0044] 1. Test drug
[0045] RPMI 1640 medium (Gibco Company), trypsin (Gibco Company); tetramethylazolazolium blue (MTT) (Suzhou Yake Chemical Reagent Co., Ltd.), super newborn bovine serum (Suzhou Yake Chemical Reagent Co., Ltd.) , Dimethyl Sulfoxide (DMSO) (Beijing Chemical Plant)
[0046] 2. Cell lines
[0047] Human gastric cancer cell line SGC-7901, human colon cancer cell line SW-480, human leukemia cell line L1210, human breast cancer cell line MCF-7, human lung adenocarcinoma cell line (SPCA-1) and human liver cancer cell line (BEL-7402 )
[0048] 3. Operation steps
[0049] Human gastric cancer cell line SGC-7901 in logarithmic growth phase, human colon cancer cell line SW-480, human leukemia cell line L1210, human breast cancer cell line MCF-7, human lung adenocarcinoma cell line (SPCA-1) and human Liver cancer cells (BEL-7402) and other 6 cancer cell lines were digest...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com