2,4'-biphenol compound with angiogenesis inhibiting effect and pharmaceutica use thereof
A technology of biphenol and compounds, which is applied in the preparation of organic compounds, organic chemistry, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
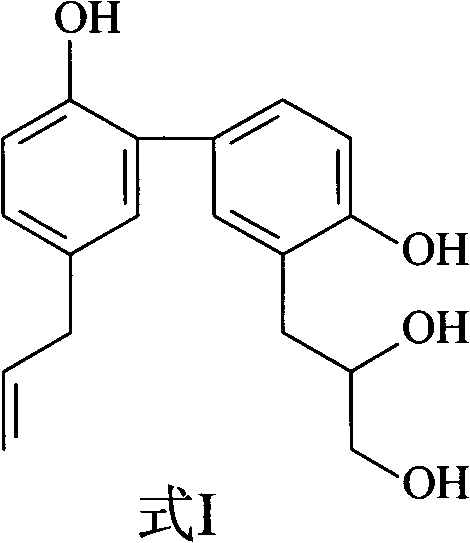
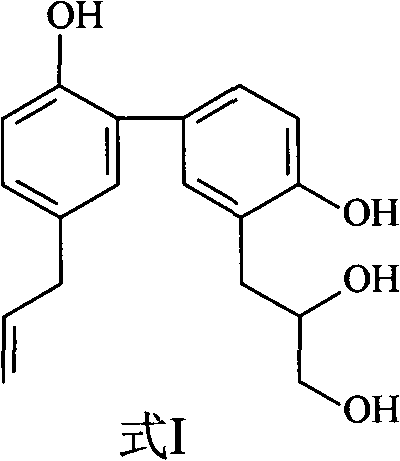
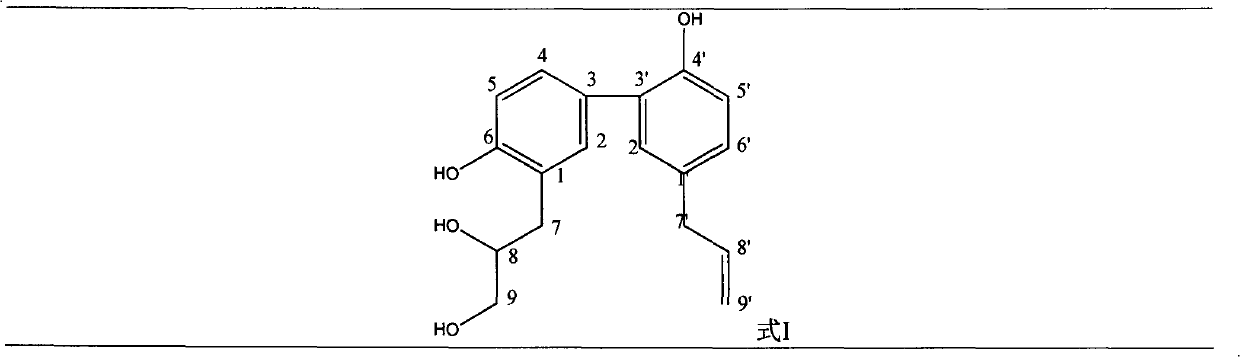
[0031] Embodiment 1: the preparation of formula I compound
[0032] 1. Take 100 kg of Magnolia officinalis medicinal material, crush it into a coarse powder, add 70% ethanol to extract, the amount of ethanol is 8 times of the weight of the medicinal material respectively, the extraction time is 2 hours each time, and the extraction times are 3 times.
[0033] 2. Filtrate the extract, combine the filtrates, and concentrate under reduced pressure at 60°C to form an extract.
[0034] 3. Add 10 times the amount of 5% sodium hydroxide solution to the extract, stir to dissolve, let it stand until the solution is clear, filter, then add hydrochloric acid to adjust the pH value to 2-3, stand overnight, filter, and get acid precipitate .
[0035] 3. The acid precipitate was washed with deionized water until neutral, dried and pulverized to obtain the precipitate.
[0036] 4. Take the precipitate, add dichloromethane to reflux and extract, the amount of solvent used is 5 times of the ...
Embodiment 2
[0043] Example 2: Inhibitory effect of the compound of formula I on the proliferation of human vascular endothelial cells.
[0044] Human umbilical cord vascular endothelial cells (HUVEC, provided by the Third School of Clinical Medicine, Peking University). Cultured in 1640 medium (Gibco company) containing 10% fetal bovine serum (FBS, Gibco company) (37 ° C, 5% CO 2 , 95% humidity), the fourth-generation cells were inoculated in a 96-well culture plate at a density of 4000 / 50 μl, and a control group, a drug group with various concentration gradients and a blank control were set, and 3 replicate holes were made in each group. When the cell growth density reached 70%, 5 μl of the compound solutions of formula I (dissolved in DMSO) with various concentration gradients were added to make the final concentrations respectively 1000 μM, 100 μM, 10 μM and 1 μM. The control group was added with 5 μl DMSO, and the culture was continued (1640 medium, 10% FBS) after 48 hours, add 20 μl...
Embodiment 3
[0045] Example 3: Inhibitory effect of the compound of formula I on the tube-forming ability of human vascular endothelial cells.
[0046] Add 200 μl of BD Matrigel to each well of a 24-well culture plate to make it polymerized into a gel, and take the fourth-generation human umbilical vein endothelial cell (HUVEC, provided by the Third Clinical Medical College of Peking University) suspension and press 3.0×10 -4 Cells / 500 μl were inoculated into a 24-well plate coated with Matrigel, and a control group and drug groups with various concentration gradients were set, and 10 μl of the compound of formula I with concentration gradients (1 μM, 5 μM, 10 μM, 50 μM, 100 μM, dissolved in DMSO), the control group was added with 10 μl of sterile DMSO, and three replicate wells were made for each group. 37°C, 5% CO 2 , and cultured at 95% humidity for 24 hours, observed the formation of vascular endothelial cell tubes under an optical microscope, and counted 4 low-magnification fields pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com