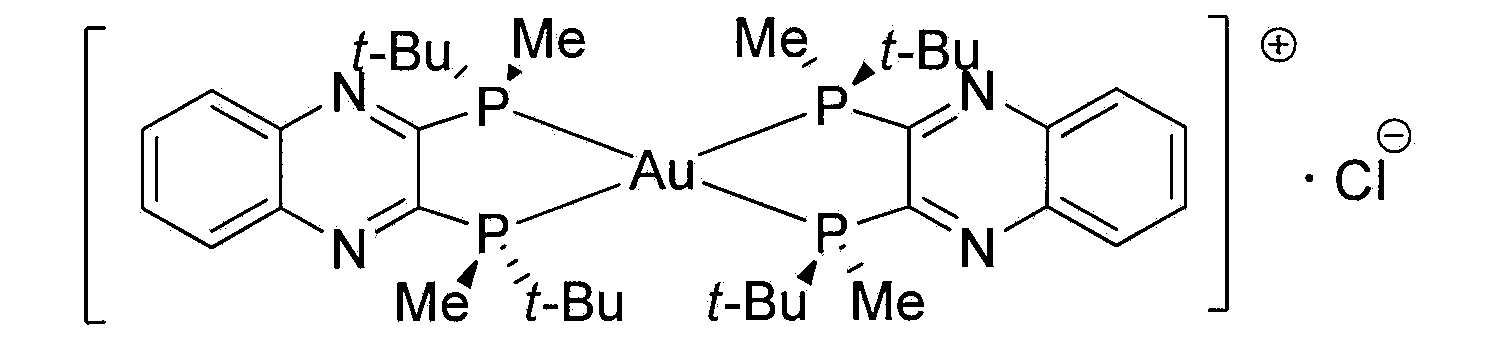
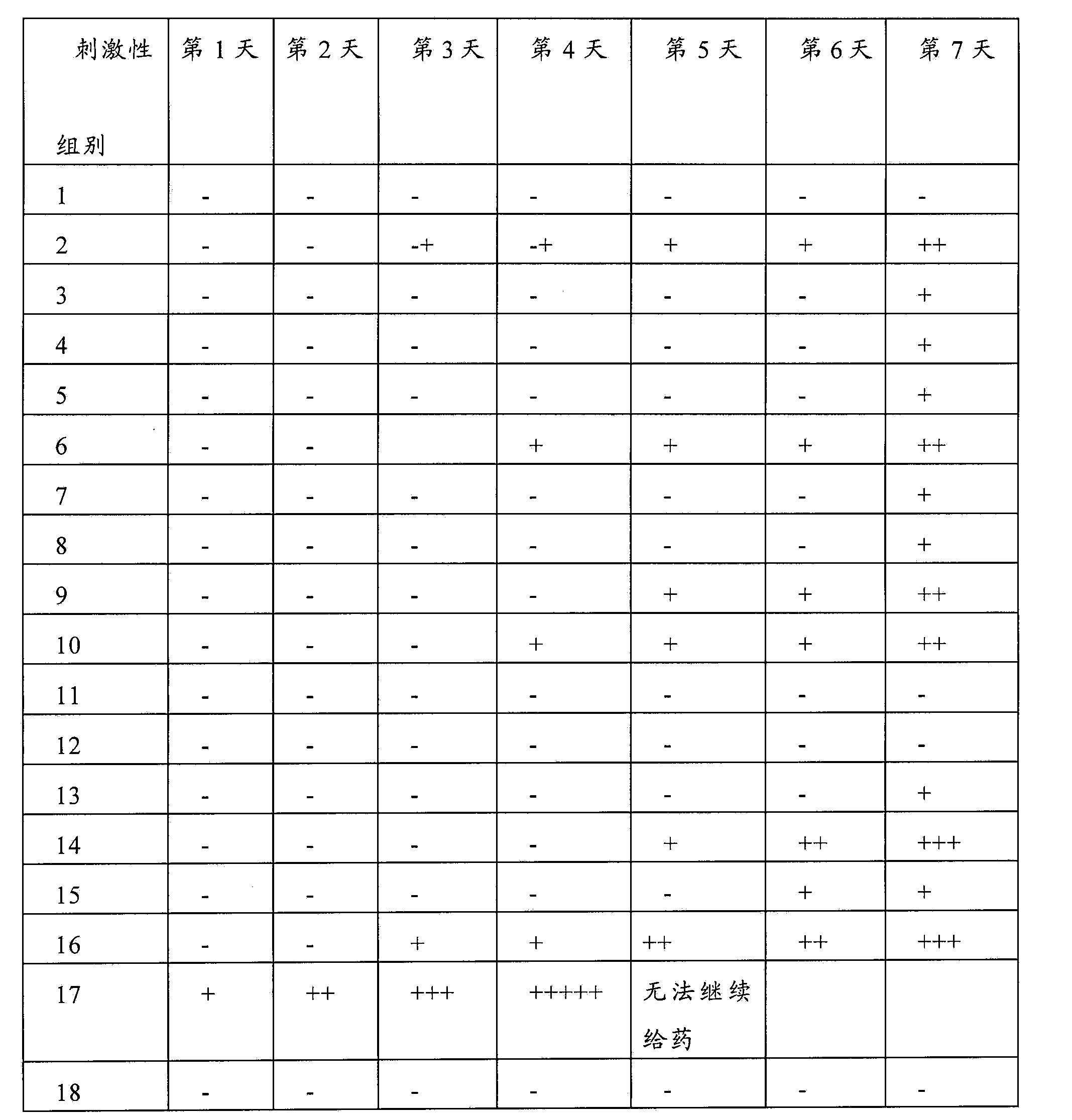
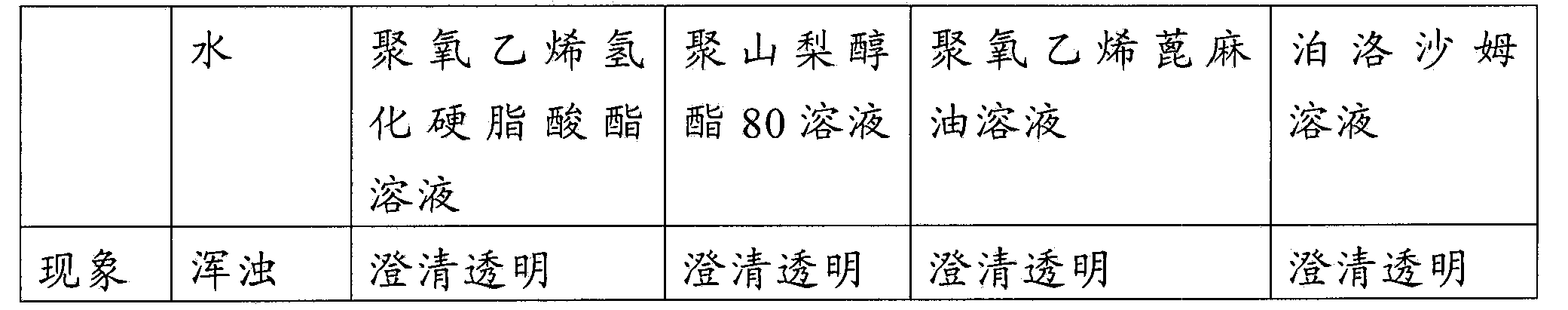
Injectable medicine composition used for resisting cancer
A technology for composition and injection, which is applied in drug combination, antineoplastic drugs, drug delivery, etc., and can solve problems such as reducing injection irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Composition prescription:
[0039] components Dosage (grams) GC-20 2 Polyoxyethylene Hydrogenated Stearate 40 Lecithin 10 Mannitol 16 prepared into 100 sticks
[0040] Preparation method: Mix GC-20, polyoxyethylene hydrogenated stearate and lecithin, under the protection of nitrogen flow, slowly add water for injection to 140ml under continuous stirring, add mannitol, dissolve, add water for injection to 200ml, 0.22 After filtering with a μm filter membrane, it is divided into vials and freeze-dried to obtain the product.
Embodiment 2
[0042] Composition prescription:
[0043] components Dosage (grams) GC-20 10 Polyoxyethylene Hydrogenated Stearate 20 glucose 10 prepared into 100 sticks
[0044] Preparation method: Mix GC-20 and polyoxyethylene hydrogenated stearate, under the protection of nitrogen flow, slowly add water for injection to 140ml under continuous stirring, add glucose, dissolve, add water for injection to 200ml, filter with 0.22μm membrane filter Afterwards, it is divided into vials, freeze-dried, and obtained.
Embodiment 3
[0046] Composition prescription:
[0047] components Dosage (grams) GC-20 0.5 Polyoxyethylene Hydrogenated Stearate 4 Lecithin 1 Mannitol 10 prepared into 100 sticks
[0048] Preparation method: Mix GC-20, polyoxyethylene hydrogenated stearate and lecithin, under the protection of nitrogen flow, slowly add water for injection to 140ml under continuous stirring, add mannitol, stir well, add water for injection to 200ml, After filtering through a 0.22 μm filter membrane, it is divided into vials and freeze-dried to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com