Calcium-sensing receptor-active compounds
A technology of compounds and stereoisomers, applied in the field of calcium-sensing receptor activating compounds, can solve problems such as women's influence, and achieve the effects of prolonging the half-life in vivo, prolonging the duration of curative effect in vivo, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
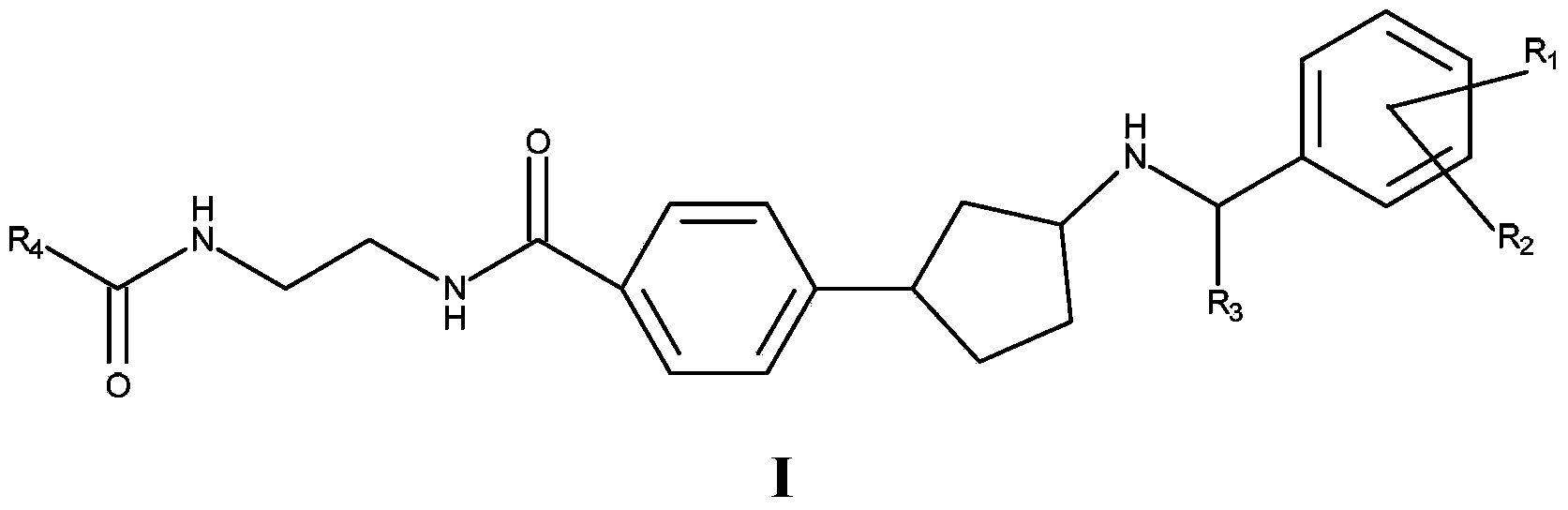
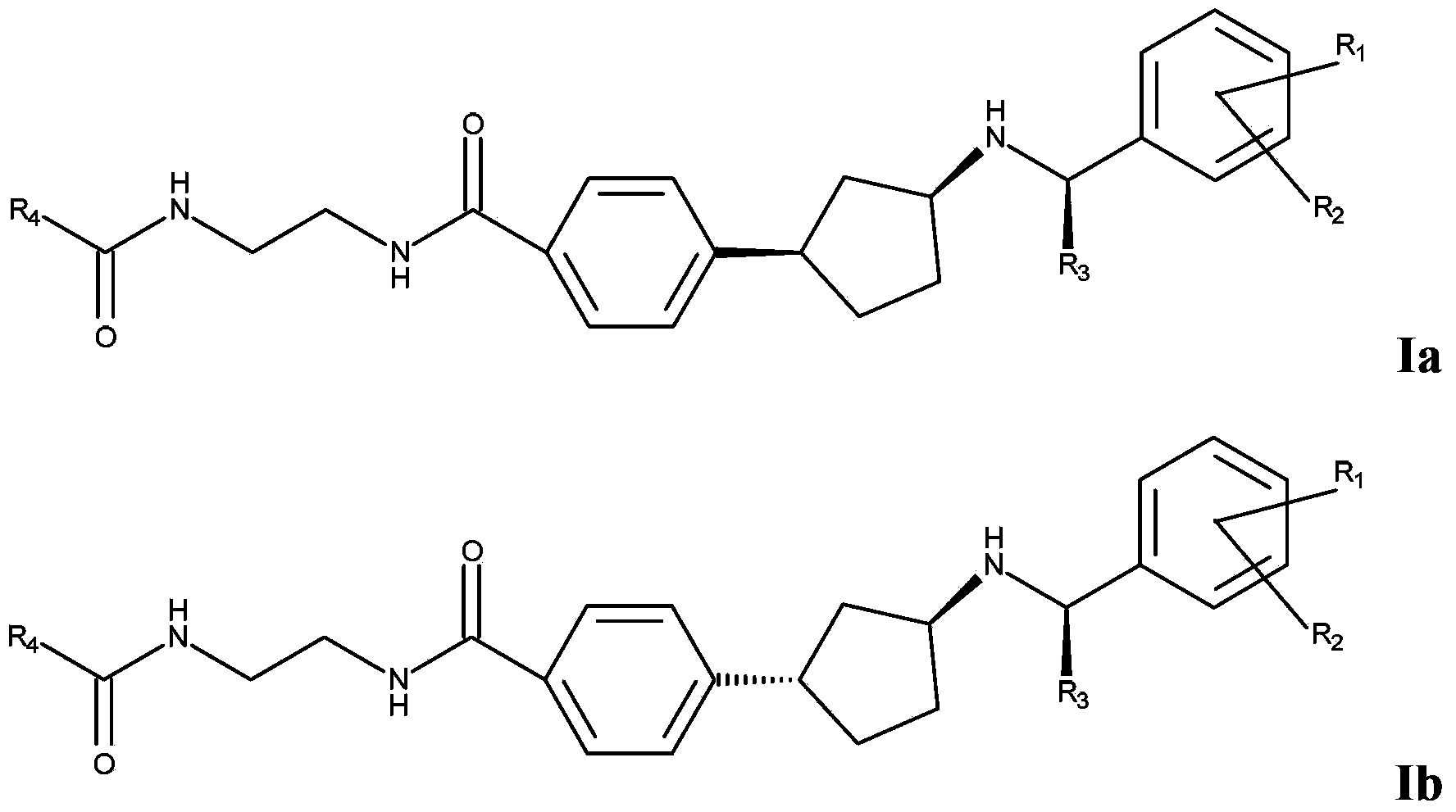
[0056] In an embodiment of the present invention, compound I is represented as Ia or Ib
[0057]
[0058] In an embodiment of the invention, R 3 stands for methyl.
[0059] In one embodiment, R 1 represents chlorine, fluorine, methoxy or ethoxy.
[0060] In one embodiment, R 2 represents chlorine, fluorine, methoxy or ethoxy.
[0061] In an embodiment of the invention, R 1 represents 4-fluoro, and R 2 represents 3-methoxy.
[0062] In one embodiment, R 1 represents chlorine, and R 2 represents hydrogen.
[0063] In an embodiment of the invention, R 4 stands for – C(O)NH 2 , C 1-4 Alkoxy, halogenated C 1-4 Alkyl, hydroxyl C 1-4 Alkyl, amino C 1-4 Alkyl, C 3-6 Cycloalkyl, C containing 1-3 heteroatoms selected from N and O 2-4 Heterocycloalkyl, C containing 1-3 heteroatoms selected from N and O 2-4 Heterocycloalkenyl, aminosulfonyl C 1-4 Alkyl, C 1-2 Alkylsulfonyl C 1-4 Alkyl, C 1-2 Alkylsulfonylamino C 1-3 Alkyl, C containing 1-3 heteroatoms selected fr...
Embodiment 1
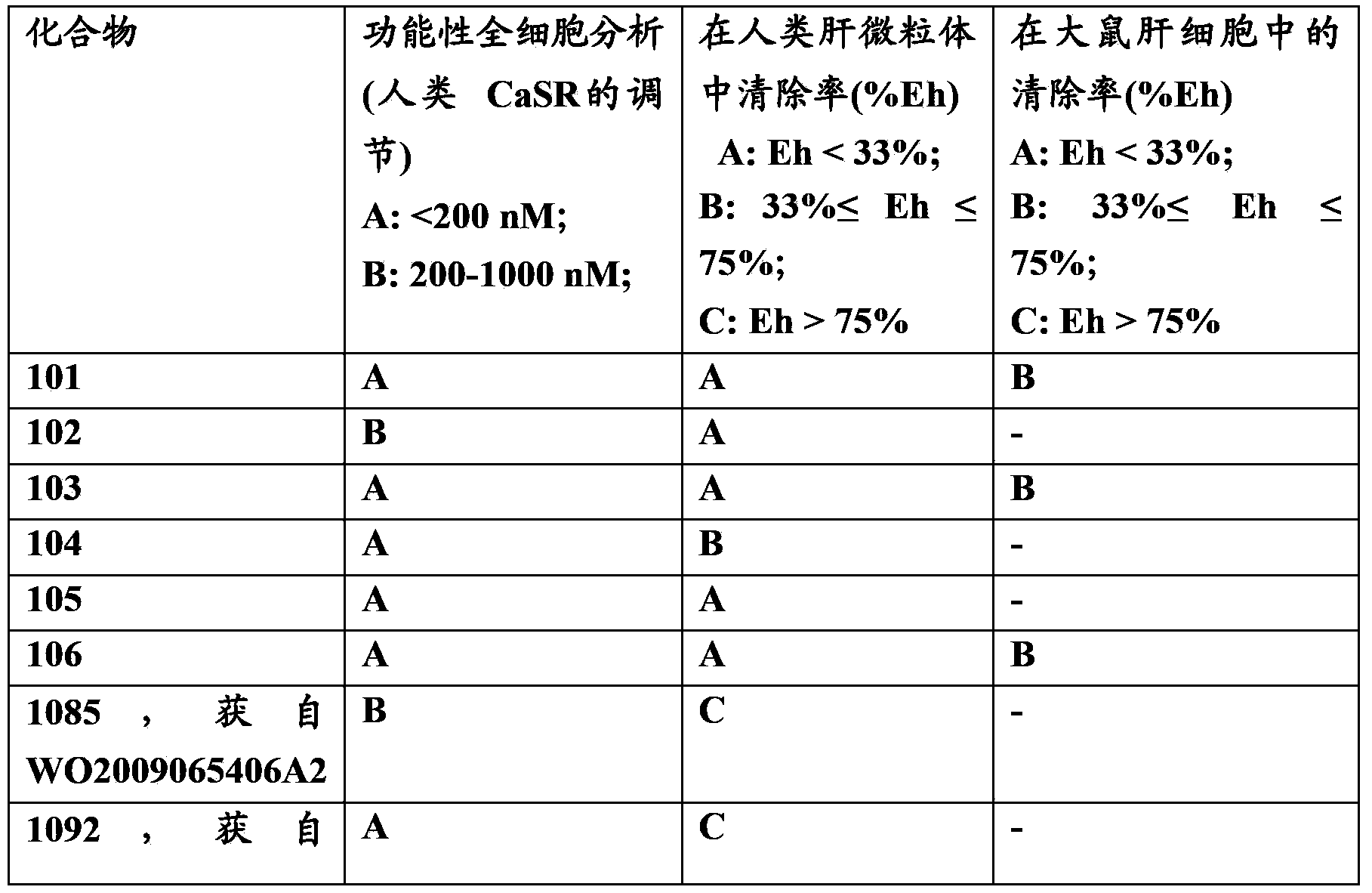
[0247] Example 1: N-[2-[[4-[(1R,3S)-3-[[(1R)-1-(4-fluoro-3-methoxy-phenyl)ethyl]-amino] Cyclopentyl]benzoyl]amino]ethyl]-1H-1,2,4-triazole-3-carboxamide (Compound 101)
[0248] To a solution of Intermediate 4 (719 mg, 1.8 mmol) in dry DMF (12 mL) were added HOBt (276 mg, 2 mmol) and EDAC (517 mg, 2.7 mmol) and the mixture was stirred at room temperature for 4 hours. Then 1H-1,2,4-triazole-3-carboxylic acid (200 mg, 1.8 mmol) was added and stirring was continued at room temperature overnight. The solvent was removed under reduced pressure and the crude product was purified by flash chromatography (30% MeOH / DCM) to give the title compound in 78% yield.
[0249] 1 H NMR(300MHz,DMSO)δ8.70(br m,1H), 8.48(br m,1H), 8.41(s,1H), 7.76(d,J=8.2Hz,2H), 7.31(d,J= 8.2Hz,2H), 7.17(dd,J=8.6,1.6Hz,1H), 7.10(dd,J=11.5,8.3Hz,1H), 6.89(ddd,J=8.1,4.4,1.8Hz,1H), 3.84(s,3H), 3.78(q,J=6.6Hz,1H), 3.46(br m,4H), 3.04–2.85(m,2H), 2.15–1.54(m,6H), 1.43–1.19(m , 4H).
Embodiment 2
[0250] Example 2: N-[2-(2,3-Dihydroxypropionylamino)ethyl]-4-[(1R,3S)-3-[[(1R)-1-(4-fluoro-3- Methoxy-phenyl)ethyl]amino]cyclopentyl]benzamide (Compound 102)
[0251] Prepared from intermediate 4 and 2,3-dihydroxypropionic acid according to GP1.
[0252] 1H NMR(300MHz,DMSO)δ8.41-8.29(m,1H), 7.94-7.85(m,1H), 7.73(d,J=8.2Hz,2H), 7.30(d,J=8.3Hz,2H) , 7.20–7.14(m,1H), 7.10(dd,J=11.5,8.3Hz,1H), 6.93–6.84(m,1H), 5.48(d,J=5.4Hz,1H), 4.64(t,J =5.6Hz,1H), 3.92–3.71(m,5H), 3.64–3.16(m,6H), 3.03–2.84(m,2H), 2.17–2.00(m,1H), 2.00–1.51(m,4H) ), 1.43–1.28 (m, 1H), 1.24 (d, J=6.5Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com