Monoclonal antibody of blood coagulation factor IX
A monoclonal antibody and blood coagulation factor technology, which is applied in the direction of coagulation/fibrinolytic factors, anticoagulation factor immunoglobulin, peptide preparation methods, etc., can solve the problems of impossibility, low safety of blood transfusion, susceptibility to infection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 Balb / c mouse immunization and splenocyte isolation
[0042] 1. Materials
[0043] Human plasma-derived FIX protein (Prospec Company), 5-week-old female Balb / c mice.
[0044] 2. Methods and results
[0045] For the first immunization, human plasma-derived FIX protein was mixed with an equal volume of Freund's complete adjuvant, and 5-week-old female Balb / c mice were injected intraperitoneally, 100 μg / mouse; after the third week, the second immunization was performed, and FIX protein was mixed with The same amount of Freund's incomplete adjuvant was mixed and injected intraperitoneally, 100 μg / mouse; after the fifth week, the third immunization was performed, this time without adjuvant, and each mouse was injected intraperitoneally with 100 μg FIX protein.
[0046] 3-5 days after the third immunization, the mice were extirpated by enucleation of eyeballs and bloodletting. Take out the mouse spleen by aseptic operation, put it in a sterilized plate, wash it...
Embodiment 2
[0047] Example 2 Fusion of mouse lymphocytes and myeloma cells and screening of hybridoma cells
[0048] 1. Materials
[0049] Mouse myeloma cell Sp2 / 0 was preserved in our laboratory, OPTI-MEM, HAT medium and fetal bovine serum were purchased from Gibco, PEG was purchased from Sigma Company, BCA protein quantification kit was purchased from Piece Company, HRP-labeled goat anti-mouse The secondary antibody was purchased from Zhongshan Jinqiao Company.
[0050] 2. Methods and results
[0051] One week before fusion, resuscitate mouse myeloma cells Sp2 / 0 at 37°C, 5% CO 2 The cells were cultured in an incubator, and the cells were passaged once 3 days before fusion. On the day of fusion, harvest myeloma cells, count, and put 5.0×10 7 Myeloma cells were washed twice with serum-free medium for later use.
[0052] Spleen cells equivalent to 1 / 2 spleen of the mouse were mixed with myeloma cells, centrifuged at 1300 rpm for 5 min, the supernatant was discarded, and removed as cle...
Embodiment 3
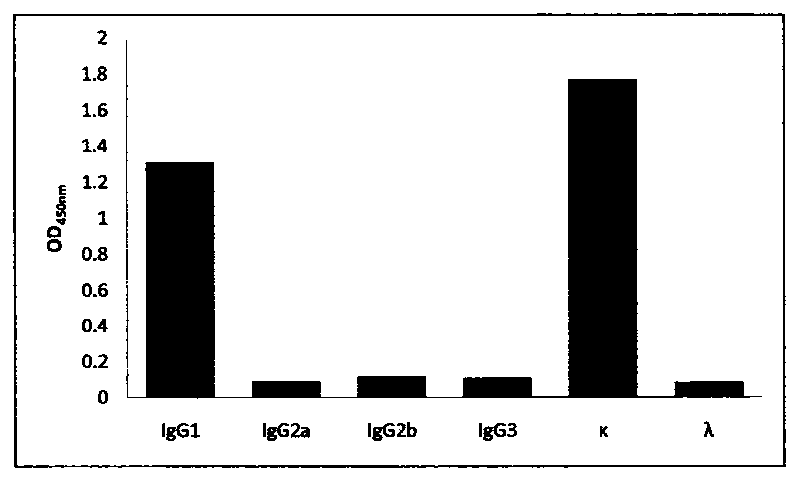
[0057] Example 3 Using immunoblotting to detect the specificity of antigen-antibody binding
[0058] 1. Materials
[0059] DAB substrate was purchased from Piece Company, and skimmed milk powder was purchased from BD Company.
[0060] 2. Methods and results
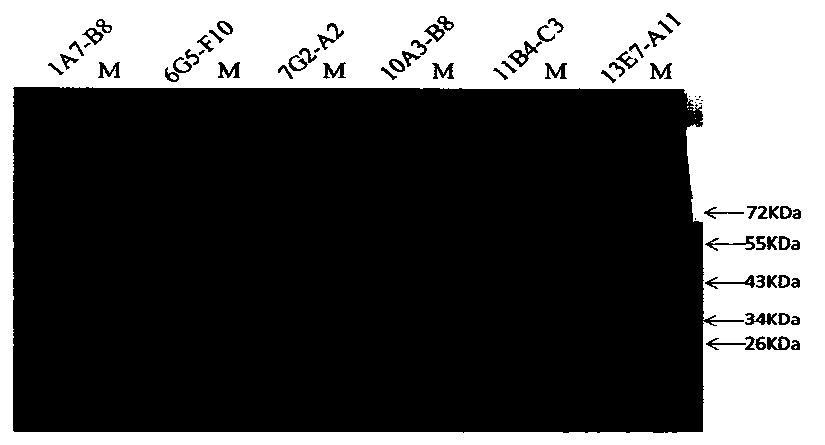
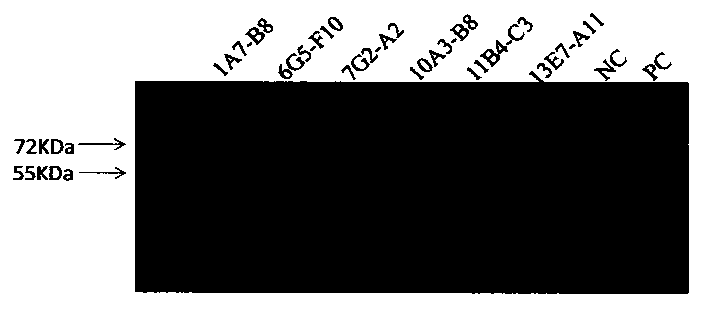
[0061] Mix 100μg of FIX protein with SDS-PAGE5×loading buffer evenly, boil at 100°C for 10min, load the sample, electrophoresis, electrotransfer to NC membrane, block with 5% skimmed milk powder at room temperature for 2h, and press the above six monoclonal antibodies with 5% skimmed milk powder respectively. Diluted at 1:1000, hybridized at room temperature for 1 h, and washed thoroughly with 0.05% PBST. HRP-goat anti-mouse secondary antibody was diluted 1:2000 with 5% skimmed milk powder, hybridized at room temperature for 1 hour, washed thoroughly with 0.05% PBST, added DAB substrate, and the results were as follows figure 1 , except the monoclonal antibody 7G2-A2 (the third lane) which has non-specific binding, all...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com