Method for synthesizing intermediate through statins
A technology for drug synthesis and intermediates, which is applied in the field of preparation of statin drug synthesis intermediates, can solve the problems of high cost, complex process equipment, and many by-products, and achieves production cost reduction, easy purification steps, and few by-products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
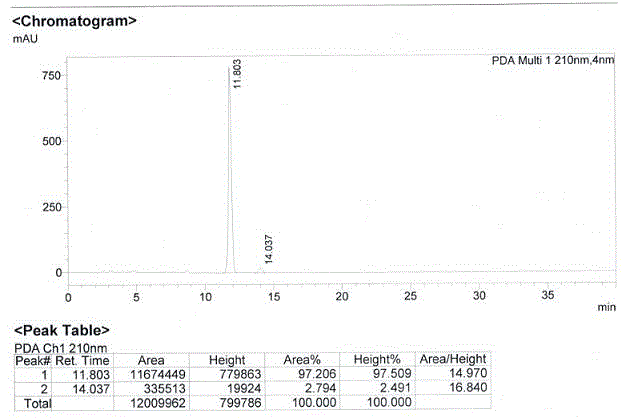
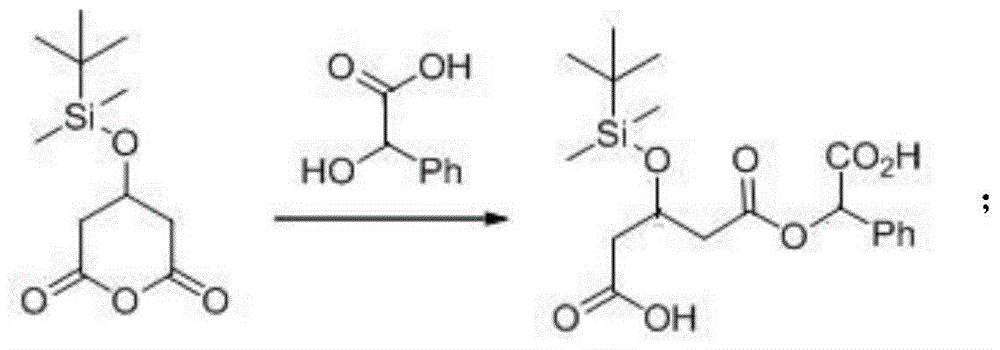
[0053] Put 8.4 g, that is, 55.2 mmol of R-mandelic acid (compound IV) into a glass reaction bottle with a capacity of 250 mL, add 40 mL of ethyl acetate (the first solvent) and stir to dissolve, then put 100 mL of toluene (the second solvent) and 10 g of 3-tert-butyldimethylsiloxoglutaric anhydride (compound III) 40.9 mmol. The mixture was stirred and heated, and refluxed for 48 hours. After the reaction, the reaction mixture was concentrated under reduced pressure to obtain an oily concentrated product. Add 40 mL of toluene solution to the oily concentrated product, heat to dissolve fully, then cool down to -20°C, crystallize unreacted R-mandelic acid, filter with suction, and discard the solid. Concentrate the filtrate under reduced pressure in vacuo until there is no solution, then add 30 mL of toluene, heat until fully dissolved, cool down to -20°C and keep crystallization for 48 hours, filter and dry to obtain 3.4 g of white solid, namely (3R)-3-tert Butyldimethylsiloxy...
Embodiment 2
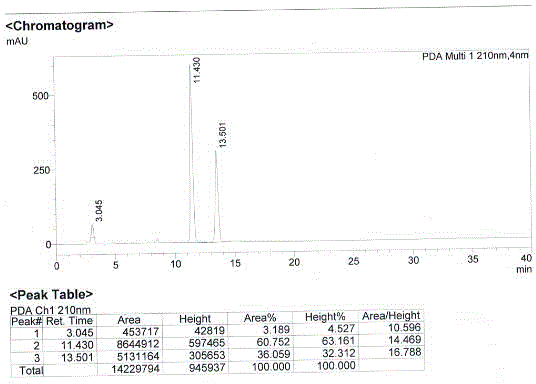
[0055] In a glass reaction bottle with a capacity of 500 mL, 12.4 g, namely 80.8 mmol of S-mandelic acid (compound VII), and 20 g of 80.8 mmol of 3-tert-butyldimethylsiloxoglutaric anhydride (compound III ), and a total of 250 mL of tetrahydrofuran was added. The mixture was stirred and heated, and refluxed for 20 hours. After the reaction, the reaction mixture was concentrated under reduced pressure to obtain an oily concentrated product. Add 50 mL of toluene solution to the oily concentrated product, heat to dissolve fully, then cool down to 15°C, keep it for 12 hours, then place it in a freezer at -20°C for 48 hours, filter and dry to obtain 27.0g of white solid , namely (3R)-3-tert-butyldimethylsiloxyglutaric acid-1-(S)-mandelate and (3S)-3-tert-butyldimethylsilyloxyglutaric acid- A mixture of 1-(S)-mandelic acid esters. By HPLC, refer to standard compounds, respectively calibrate (3R)-3-tert-butyldimethylsilyloxyglutarate-1-(S)-mandelate and (3S)-3-tert-butyldimethyl ...
Embodiment 3
[0057] Into a glass reaction bottle with a capacity of 250 mL, 6.22 g, namely 40.9 mmol of R-mandelic acid (compound IV), and 10 g of 40.9 mmol of 3-tert-butyldimethylsiloxoglutaric anhydride (compound III ), a total of 150 mL of tetrahydrofuran was added. The mixture was stirred and heated, and refluxed for 20 hours. After the reaction, the reaction mixture was concentrated under reduced pressure to obtain an oily concentrated product. Add 25mL of toluene solution to the oily concentrated product, heat, dissolve fully, cool down to 15°C, keep for 12 hours, then freeze at 0°C for 48 hours, filter and dry to obtain 9.5g of white solid, namely ( 3R)-3-tert-butyldimethylsiloxyglutaric acid-1-(R)-mandelate and (3S)-3-tert-butyldimethylsilyloxyglutaric acid-1-( R) - a mixture of mandelic acid esters. By HPLC, with reference to standard compounds, respectively calibrate (3R)-3-tert-butyldimethylsilyloxyglutarate-1-(R)-mandelate and (3S)-3-tert-butyldimethyl Siloxyglutaric acid-1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com