Method for producing proenzyme of transglutaminase through fermentation
A technology of transglutaminase enzyme and transglutaminase, which is applied in the field of fermentative production of transglutaminase proenzyme, can solve the problems of low heterologous expression and secretion, limit the application range of MTG, etc., and achieve The effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] In order to increase the production of pro-TGase, the exponential feeding method was used to realize the high-density fermentation of the mutant E62D-tag1 (the mutant constructed in the previous research process of our laboratory) to produce pro-TGase, the pro-TGase of transglutaminase.
[0055] The calculation formula of flow acceleration F:
[0056] F = μ ( VX 0 ) Y X / S ( S F - S ) exp ( μt ) - - - ( 1 ) ...
Embodiment 2
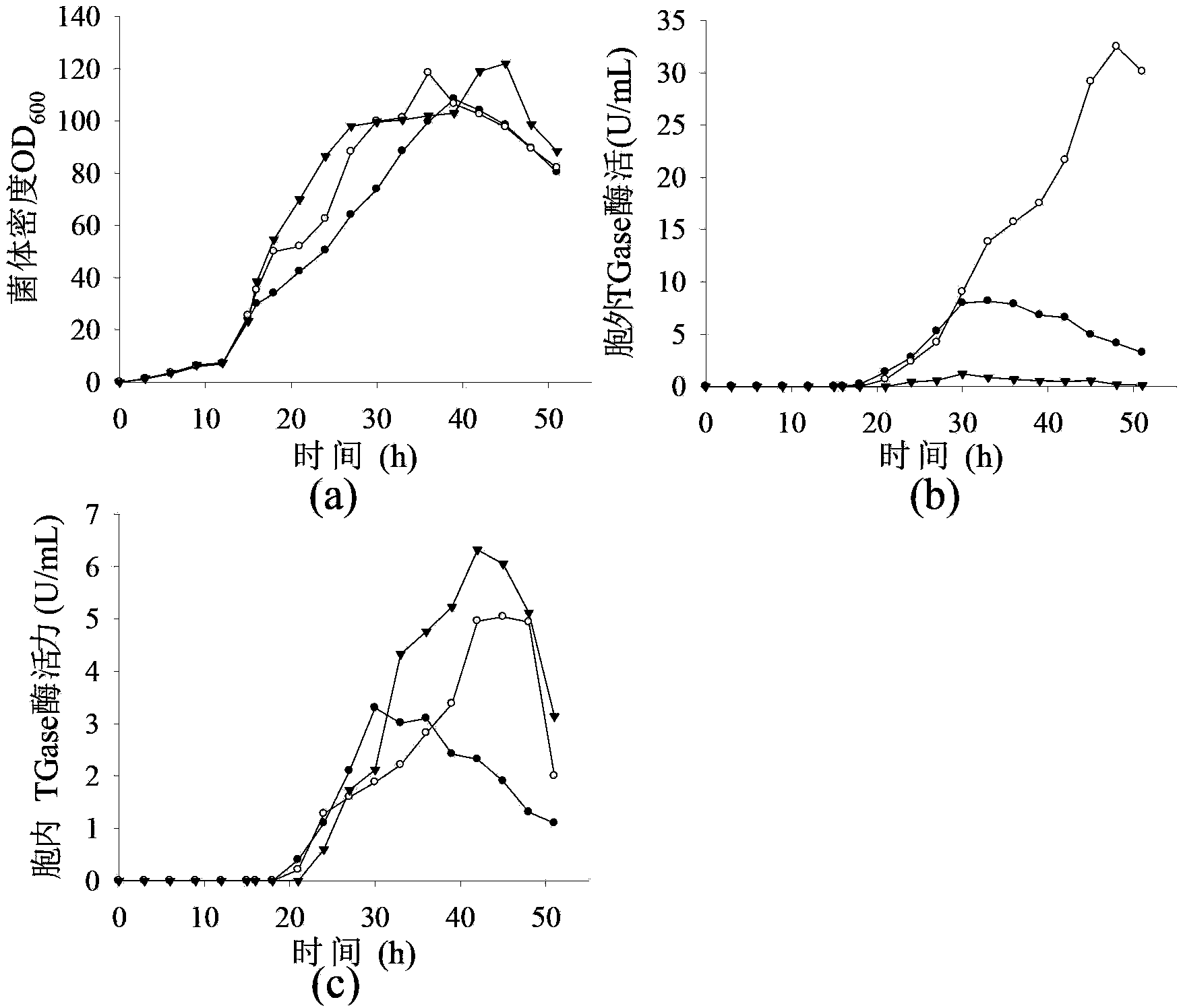
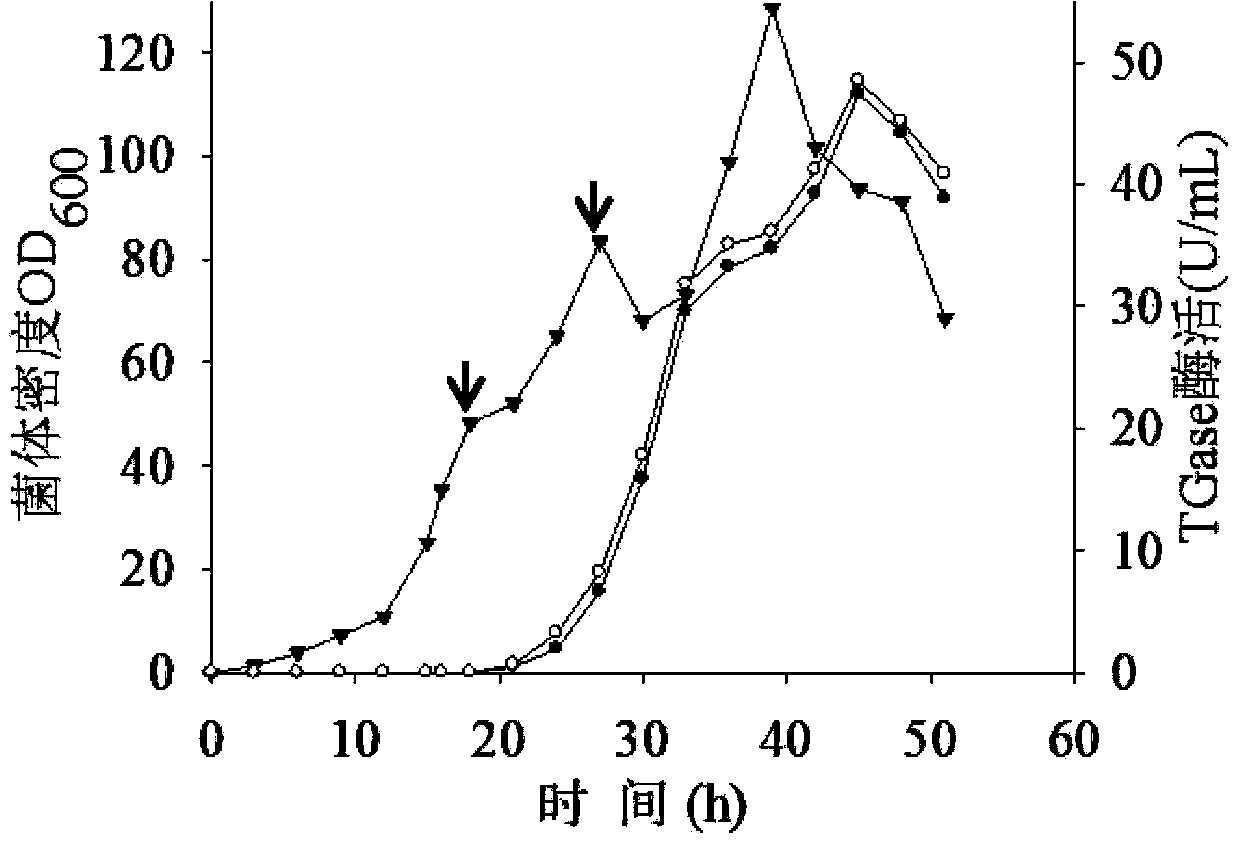
[0062] The expression of recombinant protein will put a certain pressure on the metabolism of the bacteria. Therefore, under the premise of not affecting the growth of the bacteria, choosing an appropriate induction time has an important impact on the enzyme production of the recombinant bacteria. Under high-density culture conditions, the concentration of the inducer is generally higher, but for the production of pro-TGase by recombinant Escherichia coli, a higher concentration of the inducer will cause the pro-TGase protein to accumulate in the cell and cannot be secreted out of the cell. The previous research on the fermentation of pro-TGase found that the induced bacterial concentration OD 600 When it exceeds 20, pro-TGase is accumulated in the cell and cannot be secreted outside the cell. When adding the inducer, add the final concentration of 150mmol / L glycine and 20mmol / L CaCl 2 It can promote the secretion of pro-TGase from intracellular to extracellular. Therefore, t...
Embodiment 3
[0065] Although in bacterial concentration OD 600 Higher pro-TGase production could be obtained by induction at 50, but some pro-TGase still accumulated in the cells, accounting for about 15.4% of the total pro-TGase activity. In order to promote the transport of intracellular pro-TGase to the extracellular environment, the strategy of adding glycine and calcium ions was optimized.
[0066] If the concentration of glycine and calcium ions is too high, it will affect the normal growth of bacteria, while if the concentration is too low, it will have little effect on protein secretion. Therefore, glycine and calcium ions are added to the fermentation broth twice. OD 600 When it is 50, add the inducer and the final concentration is 75mmol / L glycine and 10mmol / L CaCl 2 . OD 600 When reaching 80, add final concentration again in fermented liquid and be 75mmol / L glycine and 10mmol / L CaCl 2 . Under this culture condition, the bacterial concentration OD 600 The highest extracell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com