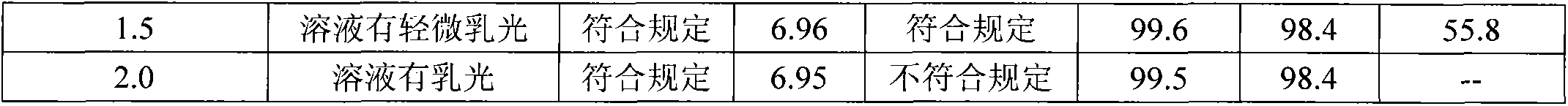Honeysuckle and baical skullcap root pharmaceutical preparation containing polyethylene glycol 12-hydroxy stearate and preparation method thereof
A technology of polyethylene glycol dodecyl stearate and pharmaceutical preparations, applied in the field of medicine, can solve problems such as being unable to meet actual clinical needs, and achieve the effects of facilitating clinical medication and promotion, and improving clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
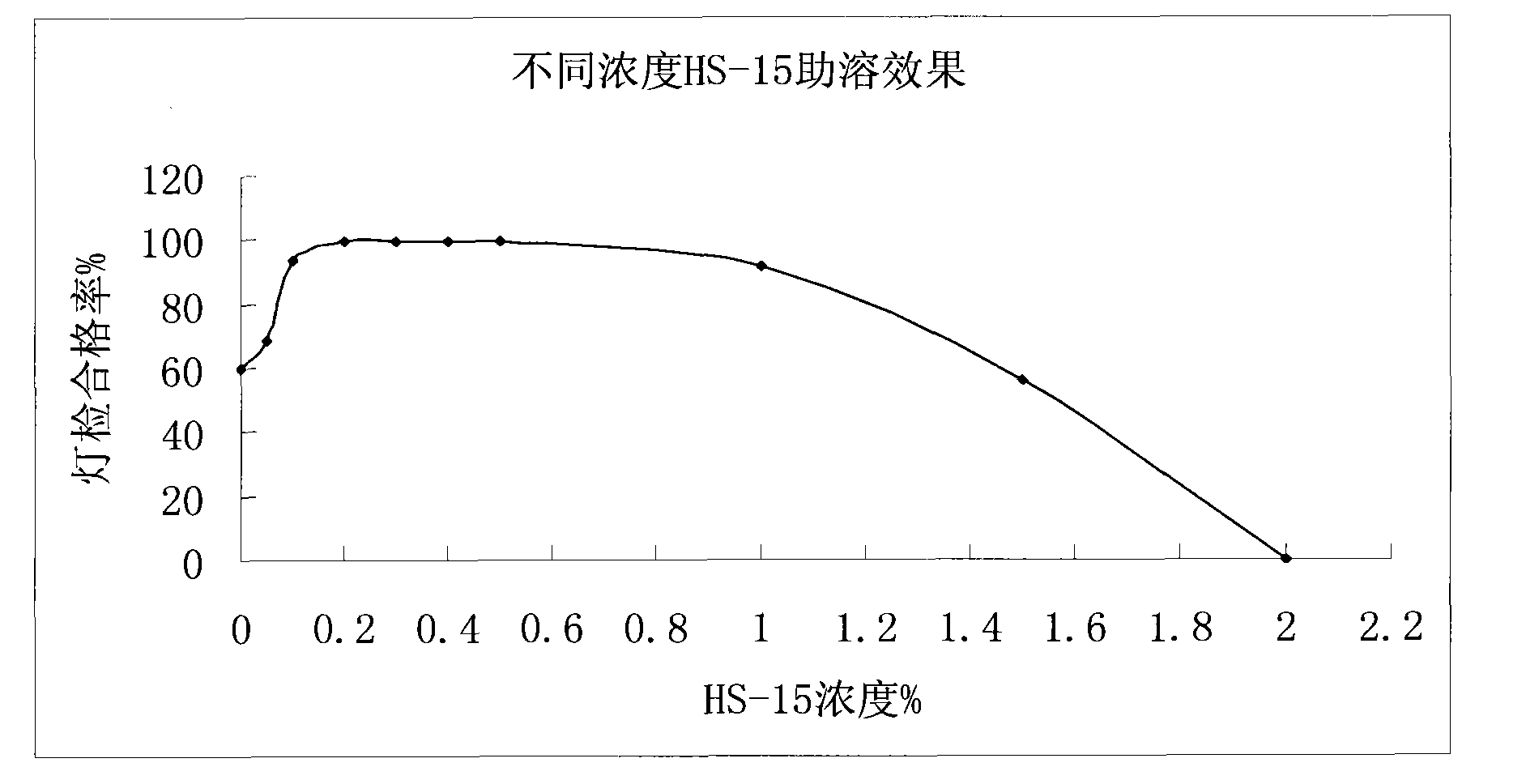
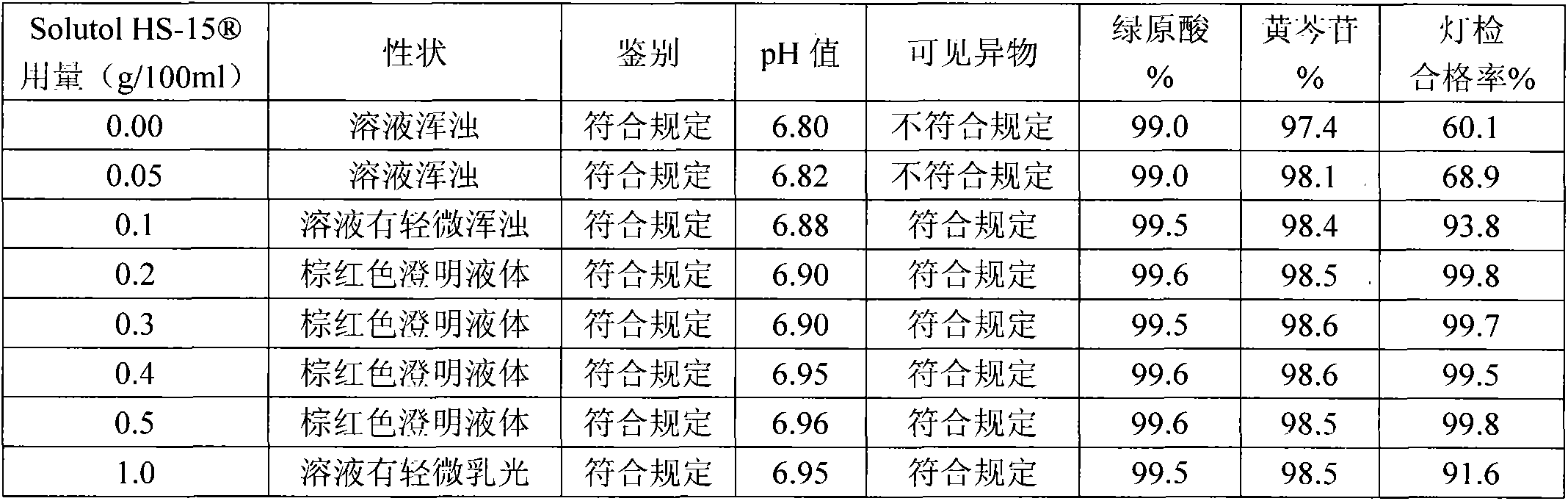
[0029] A silver yellow pharmaceutical preparation containing polyethylene glycol lauryl hydroxy stearate, which is mainly composed of honeysuckle extract, scutellaria baicalensis extract and polyethylene glycol lauryl hydroxy stearate for improving the clarity of injection The dosage of the polyethylene glycol lauryl hydroxystearate is 0.1g-1.0g / 100ml for the medicine for injection made from water for injection.
[0030] The preparation method of the silver yellow pharmaceutical preparation is as follows:
[0031] Scutellaria baicalensis extract 20.0g (calculated as baicalin)
[0032] Honeysuckle extract 12.5g (calculated as chlorogenic acid)
[0033] 2.0g
[0034] The above two flavors, respectively add water for injection and 80% sodium hydroxide solution to dissolve them, mix, add water for injection to about 980ml, adjust the pH value to 7.2 with 80% sodium hydroxide solution, add an appropriate amount of activated carbon, and heat on a water bath for 1 After hours, l...
Embodiment 2
[0036] Scutellaria baicalensis extract 20.0g (calculated as baicalin)
[0037] Honeysuckle extract 12.5g (calculated as chlorogenic acid)
[0038] 2.0g
[0039] The above two flavors, respectively add water for injection and 80% sodium hydroxide solution to dissolve them, mix, add water for injection to about 500ml, adjust the pH value to 7.2 with 80% sodium hydroxide solution, add an appropriate amount of activated carbon, and heat it on a water bath for 1 After hours, let cool, add polyethylene glycol lauryl stearate, adjust to a certain volume with water for injection. Adjust the pH value to 7.2 with 80% sodium hydroxide solution, and filter the solution through a microporous membrane. Dispense, freeze-dry, and get ready.
Embodiment 3
[0041] Polysorbate 80 (ie Tween 80) and Comparative test on the stability of solubilized Yinhuang injection
[0042] The solution stability of the Yinhuang injection prepared by the present invention is very good, which solves the problems of small white spots, precipitation and turbidity of the solution during storage and high-temperature sterilization of the Yinhuang injection. The Yinhuang injection obtained in accordance with the relevant requirements of the Chinese Pharmacopoeia 2005 edition two appendix XIXC drug formulation stability test guidelines, respectively inspected at 25 ℃ for 36 months, 40 ℃ for 6 months, 60 ℃ for 20 days The stability of the drug, the result is that the product quality is stable under the above-mentioned test conditions, and all the test indicators meet the requirements of this quality standard.
[0043] The pharmacological experiment results show that the Yinhuang injection prepared by the invention has no hemolysis, no allergy, and no irritation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com