Kit for screening protein crystals by taking salt as precipitator
A protein crystallization and precipitant technology, used in biological testing, microbial determination/inspection, material testing, etc., can solve the problems of low concentration ratio range, unfavorable protein solubility and stability, and few types of reagents. Good shape, improve the efficiency of crystallization screening, large size effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
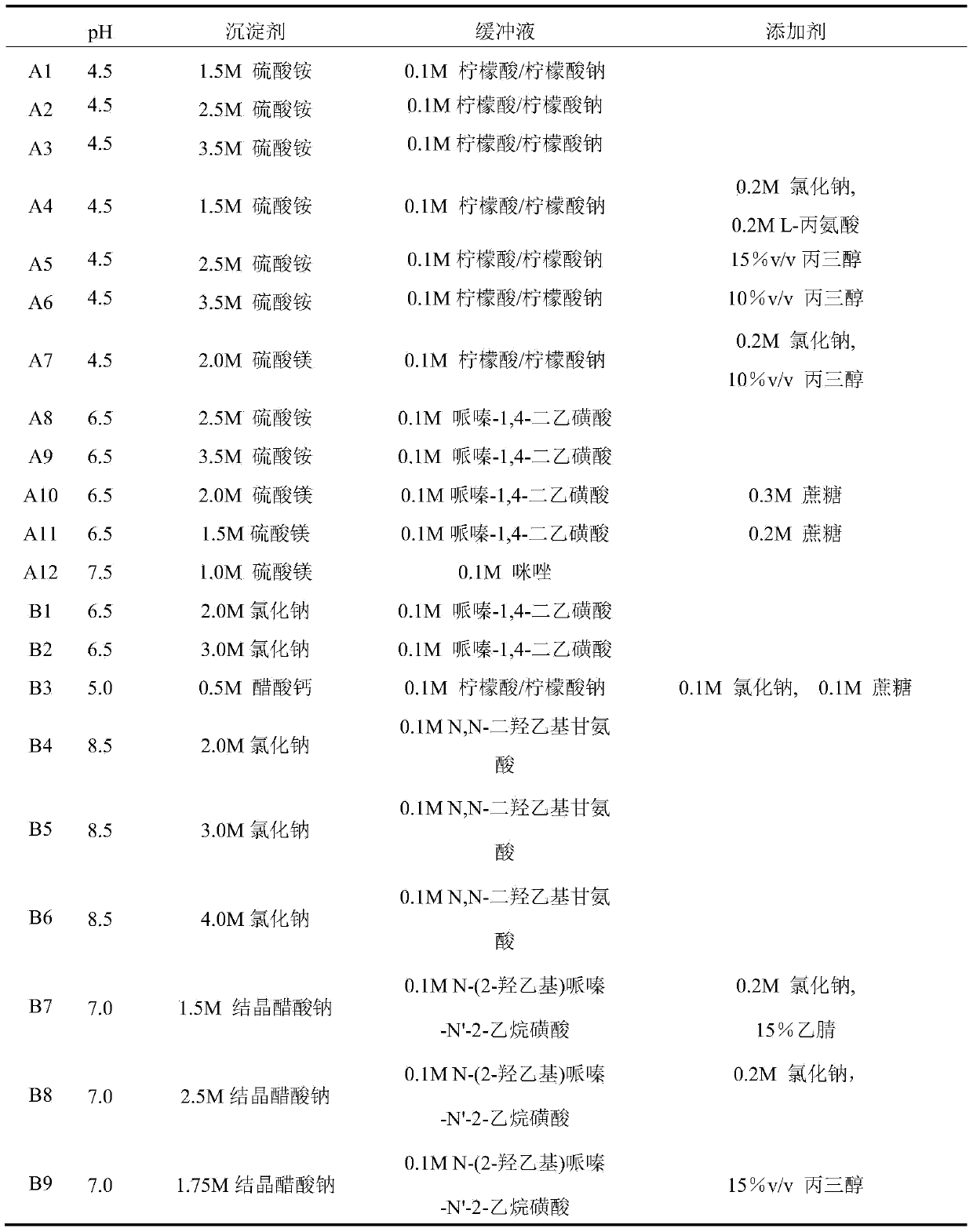
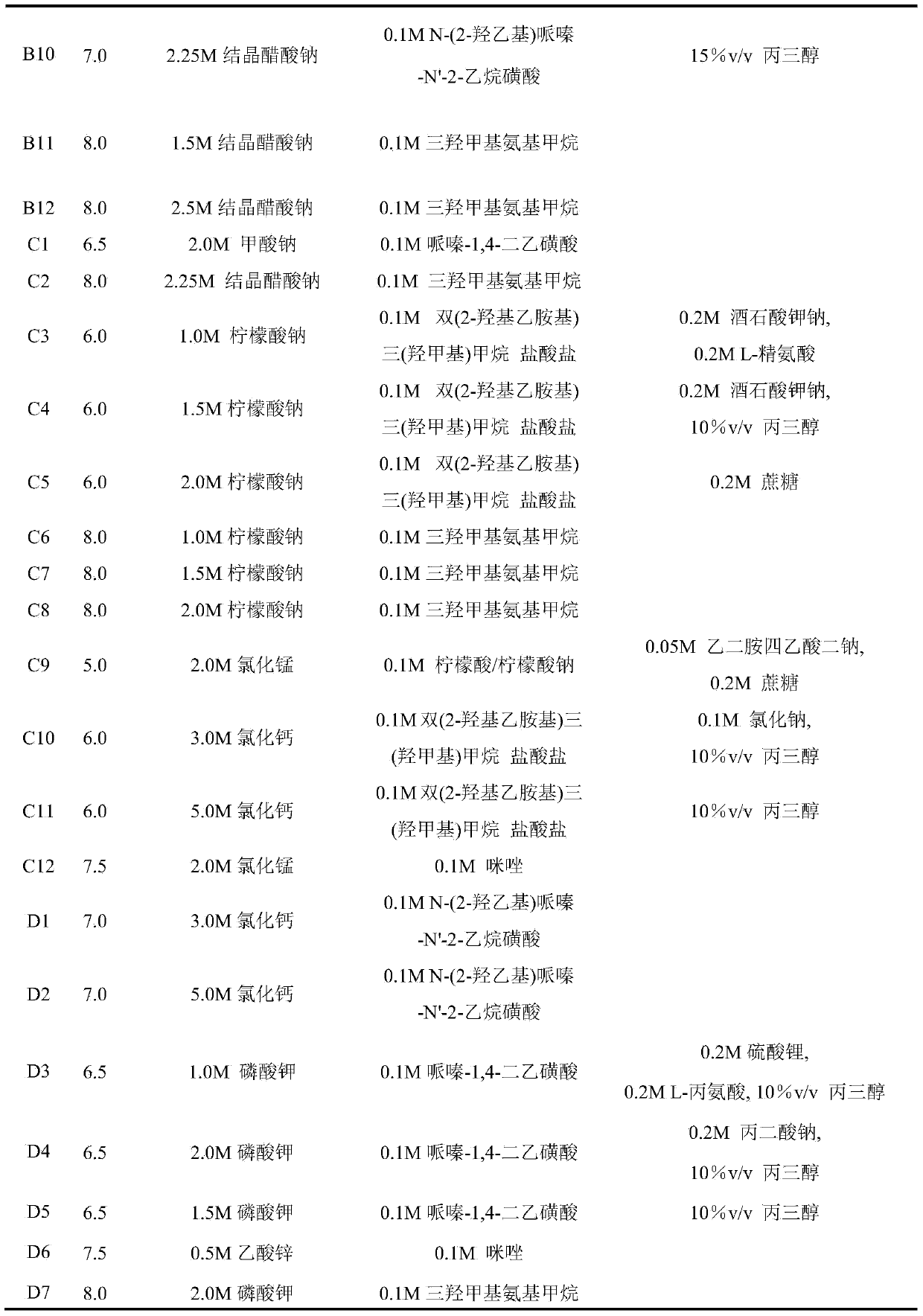
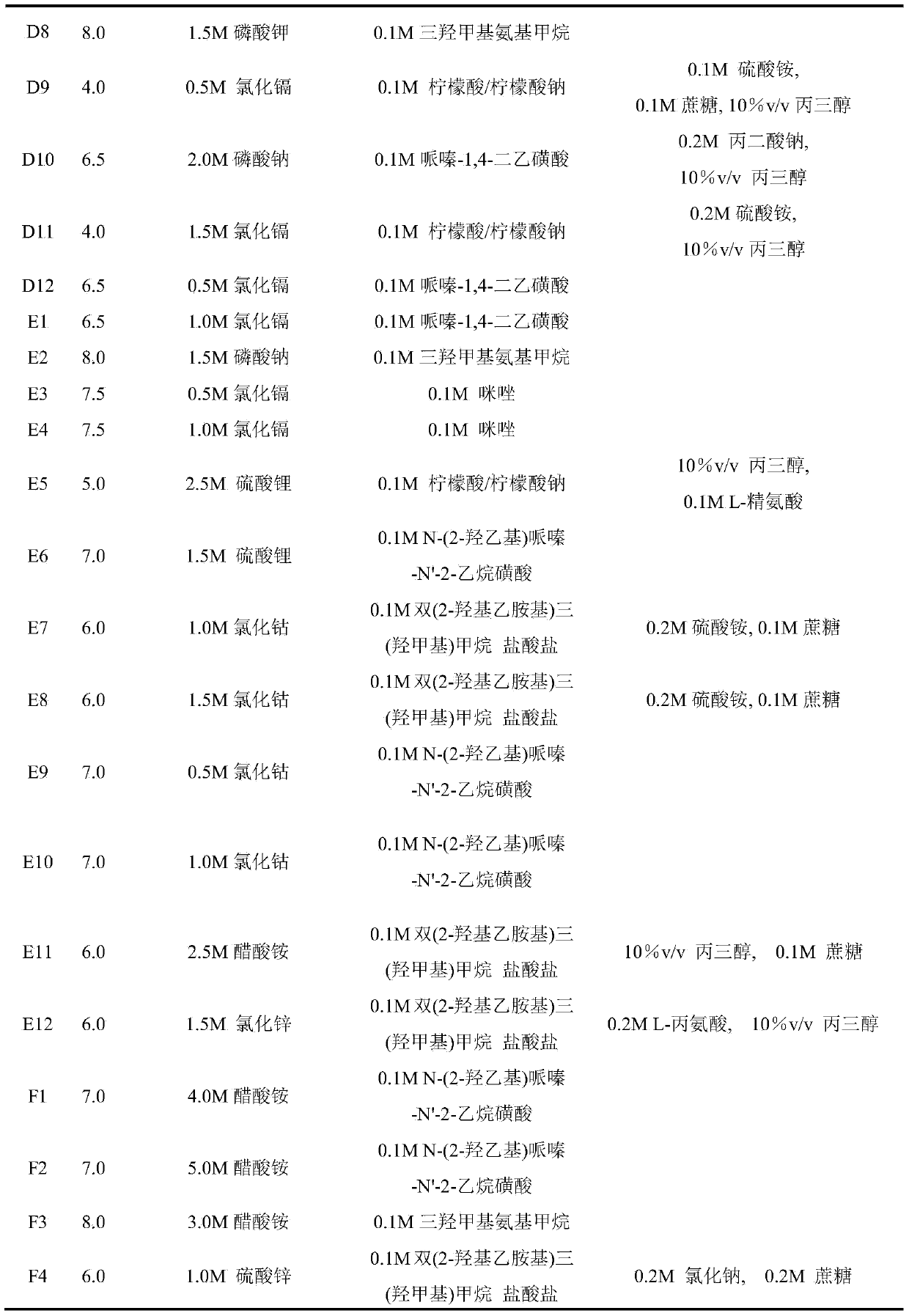
[0047] Example 1: Condition screening of lysozyme (HEWL) using the prepared Secondary Salt Screen conditions. The specific formula is shown in Table 3.
[0048]Step 1: Dissolve the six times recrystallized lysozyme (HEWL, lysozyme, lys, No. 100940) purchased from Japan Seikagaku Company in a sodium acetate buffer solution with a pH of 4.6 and a concentration of 0.1M, and prepare a concentration of 20 mg / mL lysozyme solution, filtered through a 0.22 μm sterile filter to remove impurities. It is lysozyme protein solution.
[0049] Step 2: In this example, the prepared Secondary Salt Screen96 was used to screen the crystallization conditions of lysozyme.
[0050] Step 3: Crystallization experiment Using a sitting drop plate, add 90 μL of pool solution to each deep well, use an automatic pipetting system to mix 1 μL of screening crystallization agent and 1 μL of lysozyme protein solution into a crystallization drop, and drop it in a small well. Sit in the drop well, and seal th...
Embodiment 2
[0054] Example 2: Conditional screening of proteinase K (proteinase K) using the prepared Secondary Salt Screen conditions. The specific formula is shown in Table 3.
[0055] Step 1: Dissolve proteinase K (pK, No.P6556) purchased from Sigma-Aldrich in sodium citrate buffer solution with a pH of 6.5 and a concentration of 0.5M to prepare proteinase K with a concentration of 15 mg / mL The solution was filtered through a 0.22 μm sterile filter to remove impurities. That is proteinase K protein solution.
[0056] Step 2: In this example, the prepared Secondary Salt Screen 96 was used to screen proteinase K for crystallization conditions.
[0057] Step 3: Crystallization experiment Using a sitting drop plate, add 90 μL of pool solution to each deep well, use an automatic pipetting system to mix 2 μL of screening crystallization agent and 2 μL of proteinase K protein solution into a crystallization drop, and drop it in a small well. Sit in the drop well, and seal the crystallizati...
Embodiment 3
[0061] Example 3: Concanavalin was screened using the prepared Secondary Salt Screen conditions. The specific formula is shown in Table 3.
[0062] Step 1: Dissolve concanavalin (con, No.L7647) purchased from Sigma-Aldrich in N-2-hydroxyethylpiperazine-N'-2 with a pH of 7.5 and a concentration of 0.1M - In sodium ethanesulfonate buffer, prepare a concanavalin solution with a concentration of 10 mg / mL, and filter with a 0.22 μm sterile filter to remove impurities. It is concanavalin solution.
[0063] Step 2: In this example, the prepared Secondary Salt Screen 96 was used to screen the crystallization conditions of concanavalin.
[0064] Step 3: Crystallization experiment Using a sitting drop plate, add 90 μL of pool solution to each deep well, and use an automatic pipetting system to mix 1.5 μL of screening crystallization agent and 1.5 μL of concanavalin solution into crystallization droplets , drop in a small drip hole, and seal the crystallization plate.
[0065] Step 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com