Preparation method of 4-phenylimidazole
A technology of phenylimidazole and temperature control, which is applied in the direction of organic chemistry, can solve the problem of no cost advantage, and achieve the effect of convenient product purification, less reaction steps and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
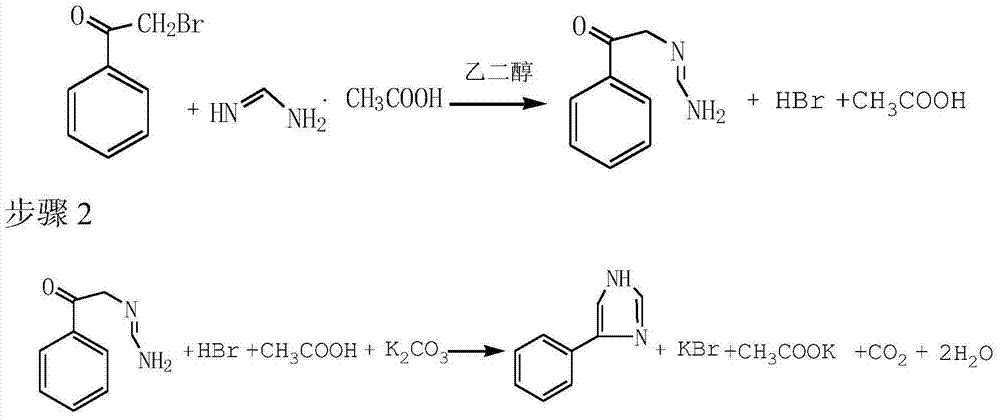
[0026] 1) Substitution: Add 200ml of ethylene glycol to a four-neck flask, raise the temperature to 50°C, slowly add 50g of α-bromoacetophenone (0.25mol) while stirring, and control the temperature at 50±2°C during the addition process. After 30 minutes of heat preservation, slowly add 55g of formamidine acetate (0.53mol) at 50±2°C for 45 minutes, and continue to react at 50±2°C for 2 hours after the addition.
[0027] 2) Cycling: Cool down to 30°C, slowly add 130g (0.94mol) of potassium carbonate, and finish adding in 1 hour, continue to keep warm at 32±2°C for 6 hours, then rapidly raise the temperature to 82±2°C, and keep warm for 5 hours. The reaction is over.
[0028] 3) Post-processing: slowly turn on the vacuum pump, and distill ethylene glycol under reduced pressure. Control the low vacuum at the beginning, increase the vacuum after the discharge is slow, maintain the vacuum degree of 15mmHg, and finally distill the ethylene glycol at 145 degrees Celsius. Steam out. ...
Embodiment 2
[0030] 1) Substitution: Add 220ml of ethylene glycol to a four-neck flask, raise the temperature to 50°C, slowly add 50g of α-bromoacetophenone (0.25mol) while stirring, and control the temperature at 55±2°C during the addition process. After 30 minutes of heat preservation, slowly add 54g of formamidine acetate (0.52mol) at 55±2°C for 60 minutes, and continue to react at 55±2°C for 2 hours after the addition.
[0031] 2) Cycling: Cool down to 30°C, slowly add 130g (0.94mol) of potassium carbonate, and finish adding in 1 hour, continue to keep warm at 32±2°C for 5 hours, then quickly raise the temperature to 86±2°C, and keep warm for 6 hours. The reaction is over.
[0032] 3) Post-processing: slowly turn on the vacuum pump, and distill ethylene glycol under reduced pressure. Control the low vacuum at the beginning, increase the vacuum after the discharge is slow, maintain the vacuum degree of 15mmHg, and finally distill the ethylene glycol at 148 degrees Celsius. Steam out. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com