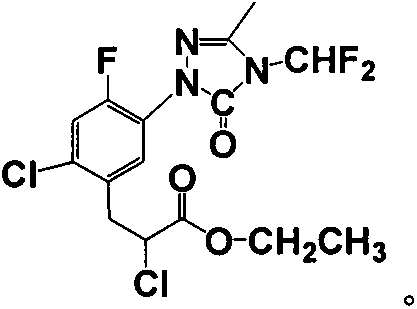
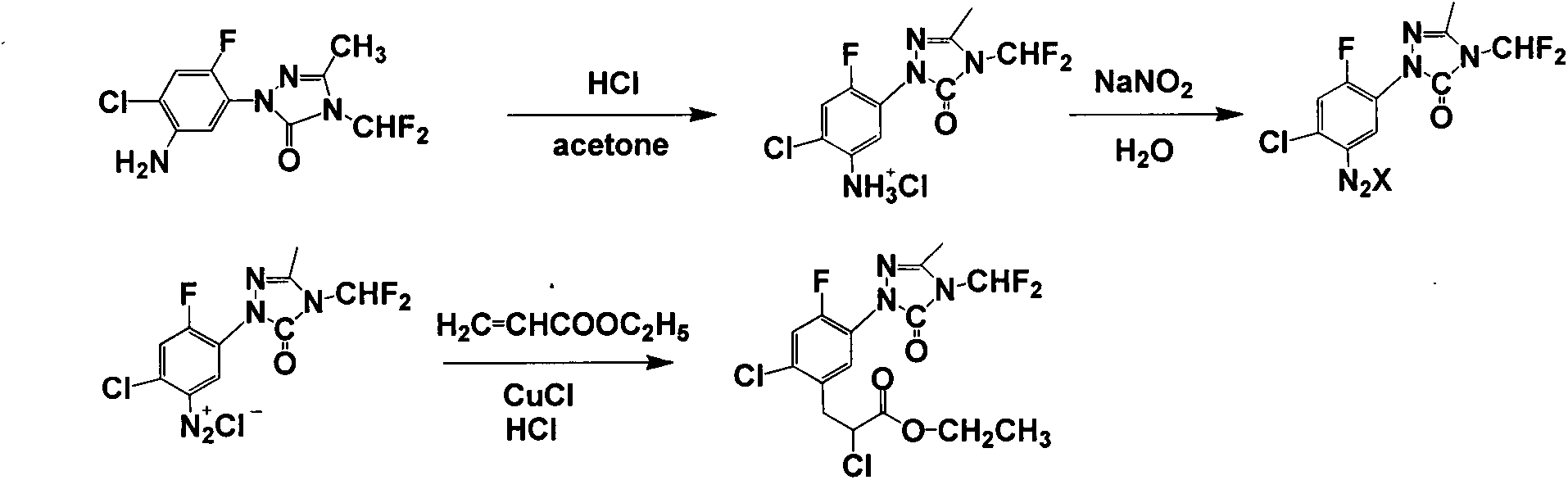
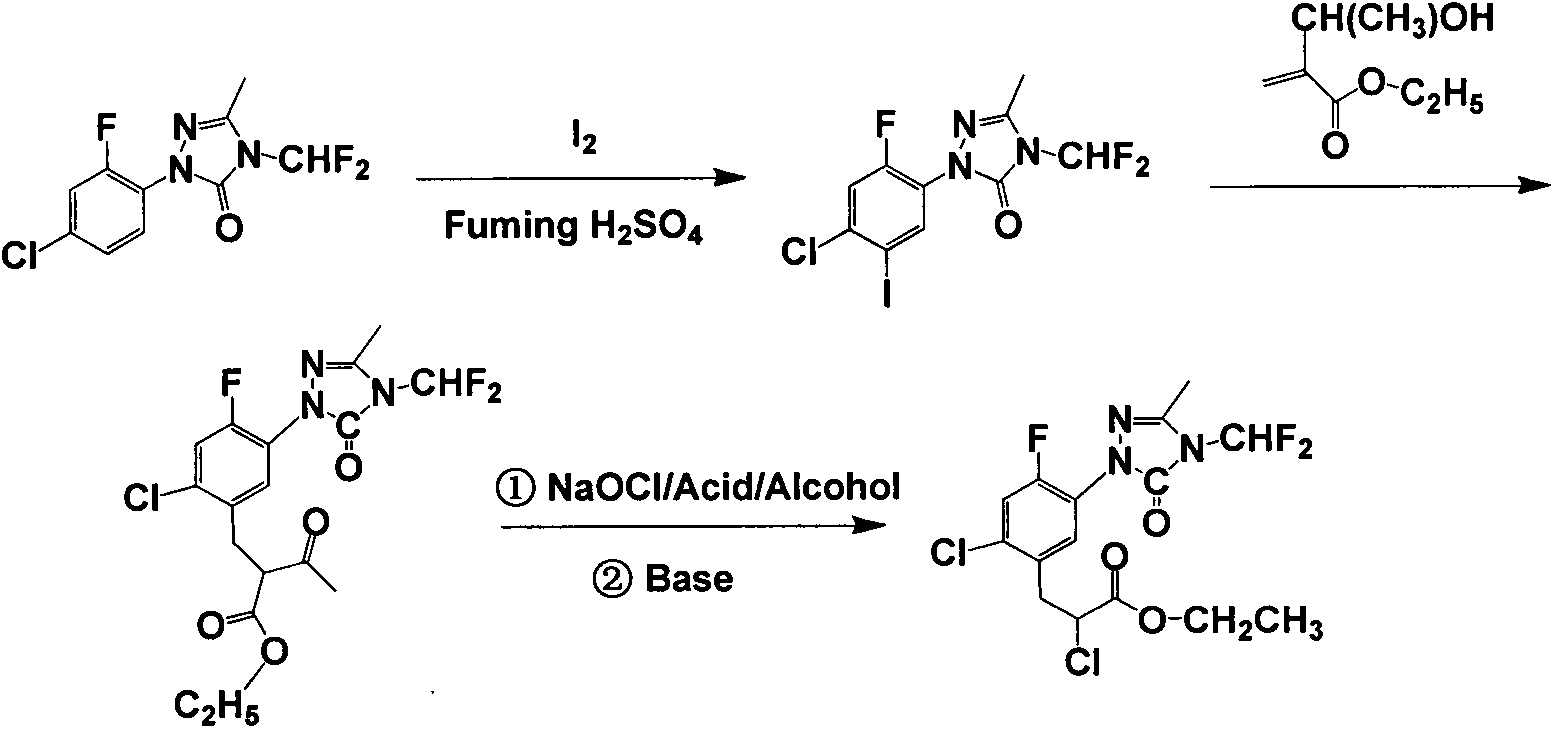
Carfentrazone-ethyl synthesis method
A synthetic method and technology of metofen, which is applied in the field of chemistry and can solve problems such as complicated operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add acetonitrile (32.4g, 790mmol) in 250mL there-necked flask, logical N 2 , cooled to -5-0°C, add 1-(5-amino-2-fluoro-4-chlorophenyl)-3-methyl-4-difluoromethyl-1H-1,2,4-triazole Lin-5-ketone (10.5g, 35.9mmol) and cuprous chloride (2.50g, 25.1mmol), add concentrated hydrochloric acid (11.8g, 120mmol) dropwise while stirring, after 20min, add ethyl acrylate ( 49.4g, 494mmol), and then controlled the temperature at 0-5°C, added dropwise tert-butyl nitrite (5.6g, 53.9mmol), added 2.5h, stirred for 1h, recovered the solvent and unreacted ethyl acrylate by atmospheric distillation Esters were diluted with ethyl acetate, and cuprous chloride was recovered by filtration. Finally, impurities were evaporated under conditions of temperature 110-125° C. and pressure 15-20 mmHg to obtain 15.2 g of the product mefentrazone, with a yield of 103%. The content is 91.8%.
Embodiment 2
[0020] Add acetonitrile (32.4g, 790mmol) in 250mL there-necked flask, logical N 2 , cooled to -5-0°C, add 1-(5-amino-2-fluoro-4-chlorophenyl)-3-methyl-4-difluoromethyl-1H-1,2,4-triazole Lin-5-ketone (10.5g, 35.9mmol) and cuprous chloride (2.50g, 25.1mmol), add concentrated hydrochloric acid (11.8g, 120mmol) dropwise while stirring, after 20min, add ethyl acrylate ( 49.4g, 494mmol), and then controlled the temperature at 0-5°C, added dropwise tert-butyl nitrite (5.6g, 53.9mmol), added 2h, stirred for 1h, recovered the solvent and unreacted ethyl acrylate by atmospheric distillation , then dilute with ethyl acetate, filter and recover cuprous chloride, and finally evaporate impurities at a temperature of 110-125°C and a pressure of 15-20mmHg to obtain 14.7g of the product mefentrazone, with a yield of 99.3%. 92.0%.
Embodiment 3
[0022] Add acetonitrile (32.4g, 790mmol) in 250mL there-necked flask, logical N 2 , cooled to -5-0°C, add 1-(5-amino-2-fluoro-4-chlorophenyl)-3-methyl-4-difluoromethyl-1H-1,2,4-triazole Lin-5-ketone (10.5g, 35.9mmol) and cuprous chloride (2.50g, 25.1mmol), add concentrated hydrochloric acid (11.8g, 120mmol) dropwise while stirring, after 20min, add ethyl acrylate ( 49.4g, 494mmol), and then control the temperature at 0-5°C, dropwise add tert-butyl nitrite (5.6g, 53.9mmol), add 3.5h, stir for 1h, recover the solvent and unreacted ethyl acrylate by atmospheric distillation Esters were diluted with ethyl acetate, and cuprous chloride was recovered by filtration. Finally, the impurity was evaporated at a temperature of 110-125° C. and a pressure of 15-20 mmHg to obtain 15.5 g of the product mefentrazone, with a yield of 105%. The content is 91.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com