Method for detection and evaluation of anti-virus-infection activity
An anti-viral and active technology, applied in the field of antibody engineering and anti-viral medicine, can solve the problems that the application efficiency and value of cell infection models and animal protection models are yet to be seen, there are no cell infection and animal protection models, and the operation process is simple , Good reproducibility of results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
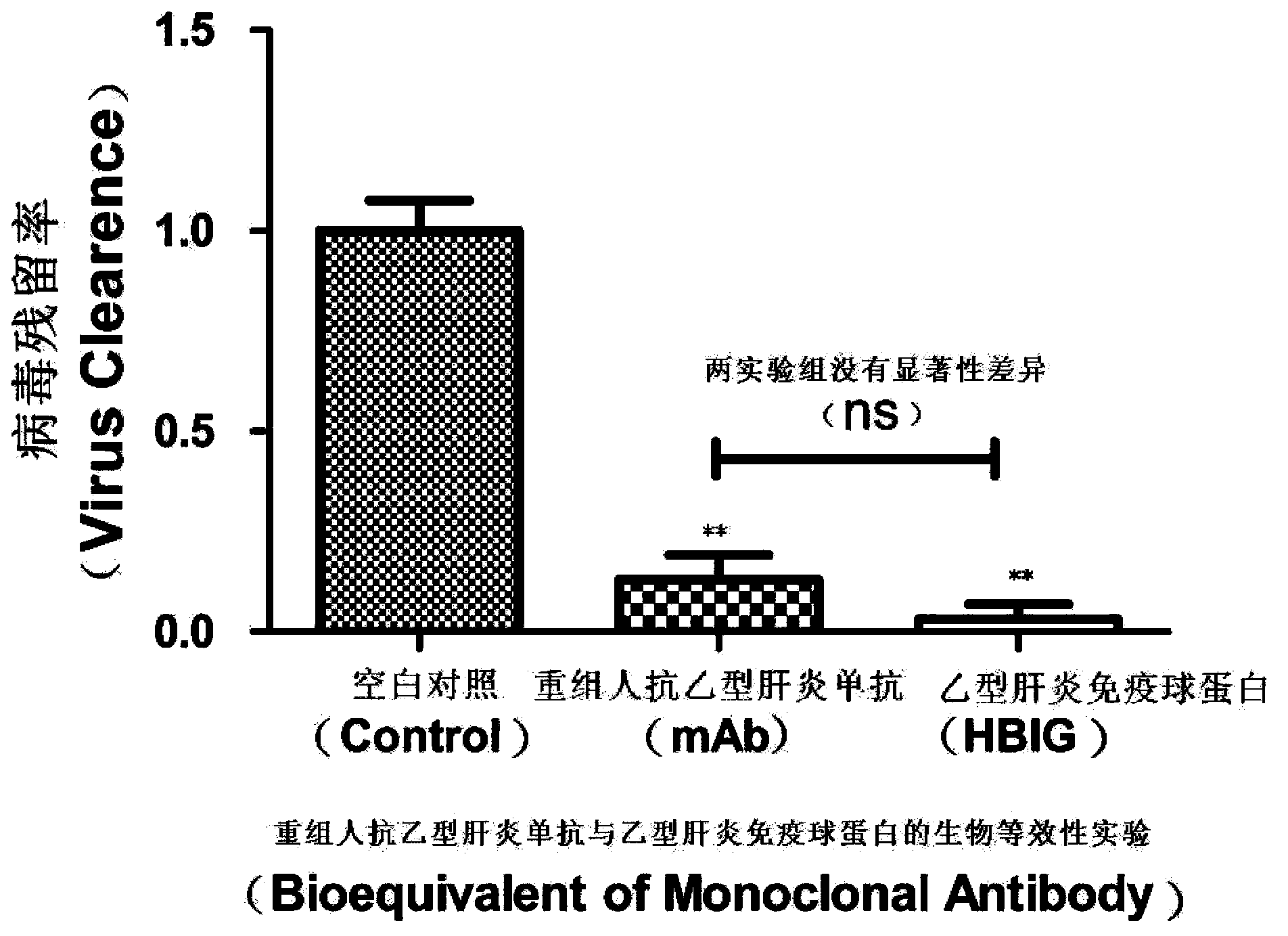
[0046] Example 1: Anti-infective ability of HBV monoclonal antibody and HBIG
[0047] The antiviral activities of the HBV monoclonal antibody and HBIG were detected respectively by using the detection method established in the present invention. HBIG is the human anti-hepatitis B virus globulin produced by Zhongsheng Group, and the humanized anti-hepatitis B virus monoclonal antibody is provided by the Institute of Viral Disease Prevention and Control, Chinese Center for Disease Control and Prevention.
[0048] The specific detection steps are:
[0049] (1) Take isolated blood samples and separate to obtain serum samples of pathogens;
[0050] (2) After mixing the pathogen serum sample with reagent A and reagent B at a ratio of 1:4.5:4.5, add 0.5% Volume test molecules (divided into HBV monoclonal antibody group and HBIG group);
[0051] (3) Stand at room temperature for 3 hours, then centrifuge at 10,000 rpm for 30 minutes;
[0052] (4) The supernatant of the centrifuged m...
Embodiment 2
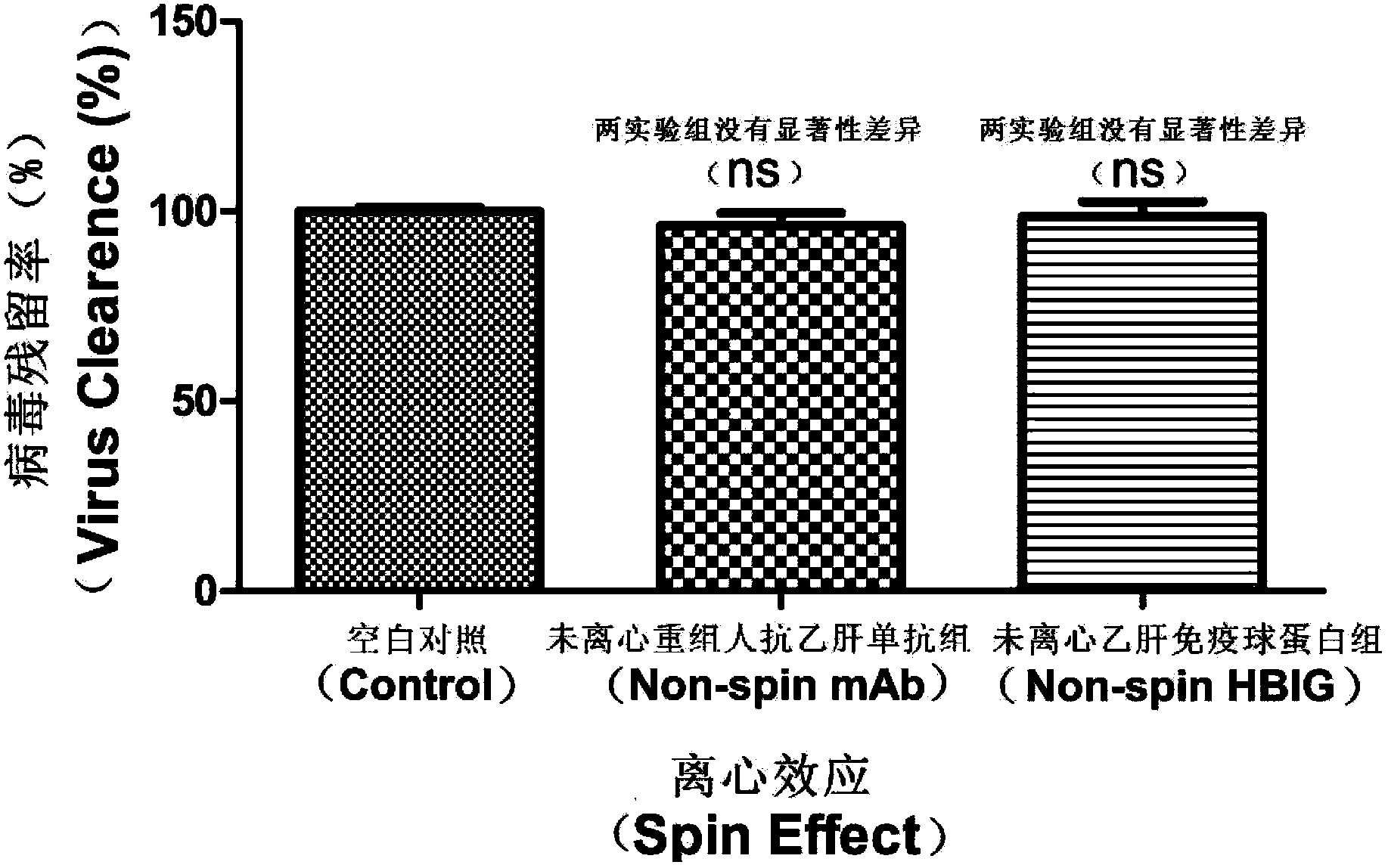
[0055] Example 2: The antiviral ability of HBIG and anti-HBV monoclonal antibody is related to the formation and deposition of viral complexes
[0056] figure 2 It is the result of the sedimentation specificity experiment.
[0057] Viral DNA was detected according to the method of Example 1, except that serum samples were treated with HBV monoclonal antibody and HBIG without sedimentation treatment, and viral DNA was detected.
[0058] The experimental results showed that the HBV monoclonal antibody BC1 and HBIG in the non-sedimentation treatment group could not clear the virus from the serum. The formation and detection of sedimentary virus complexes are necessary conditions for detection.
Embodiment 3
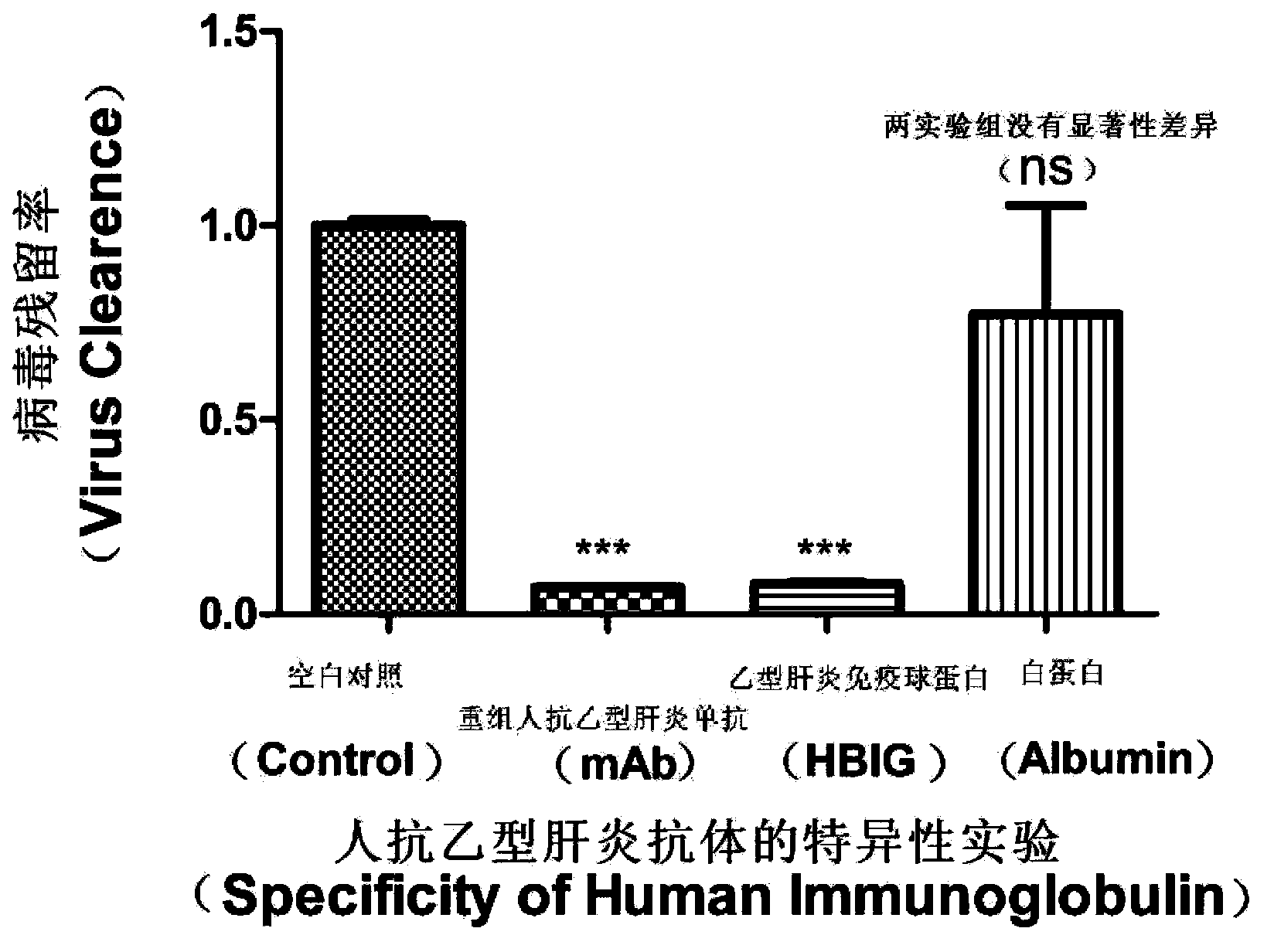
[0059] Embodiment 3 Specificity experiment of human HBV monoclonal antibody BC1
[0060] image 3 It is a specific experiment for human HBV monoclonal antibody BC1.
[0061] Viral DNA was detected according to the method in Example 1.
[0062] The results showed that the ability of HBV monoclonal antibody to neutralize the virus was 93% (P=0.0001), which was not significantly different from the ability of HBIG to neutralize the virus. The virus clearance ability of HBIG and anti-HBV monoclonal antibody BC1 was specific compared with human serum albumin control.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com