Salbutamol sulphate inhalation aerosol and preparation method thereof
A technology of salbutamol sulfate aerosol and salbutamol sulfate, which is applied in aerosol delivery, pharmaceutical formulations, respiratory diseases, etc., can solve the problem of no HFA aerosol, and achieve no toxic side effects, stable process and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Prescription
[0046]
[0047] (2) Preparation method
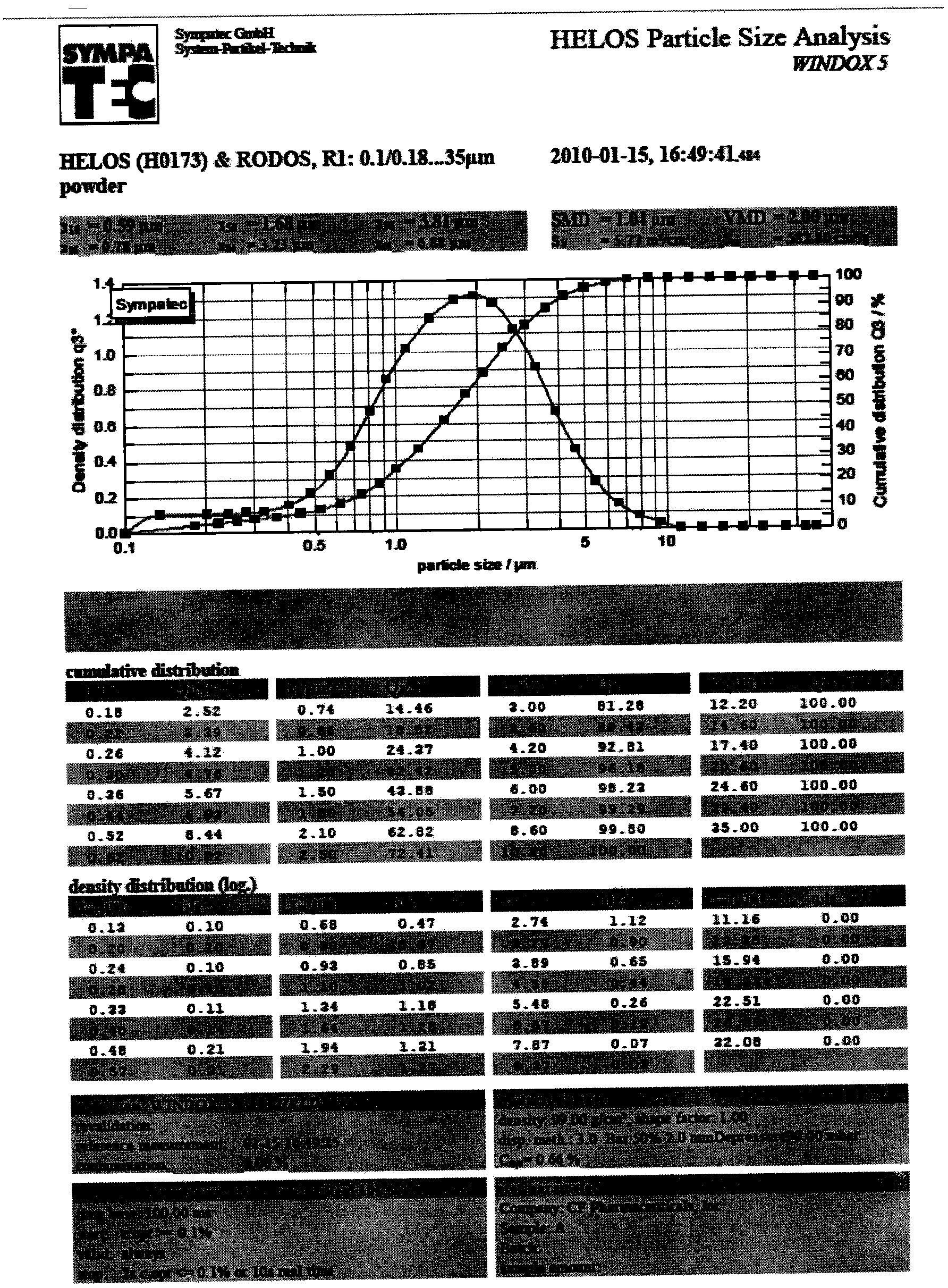
[0048] Mix albuterol and 2.5mol / L sulfuric acid evenly and pump into the reaction kettle, control the reaction temperature to 15°C, maintain a stirring speed of 900rpm / min, and control the reaction time for 10min. Filter the medicinal liquid and dry the fine salbutamol sulfate powder that obtains, see attached through powder electron microscope observation result. figure 1 The results of the particle size data of the pulverized albuterol sulfate measured by laser particle size analyzer are shown in the appendix figure 2 .
[0049] Take 2.84g of salbutamol sulfate, add it to 39.27g of absolute ethanol to dissolve, add 14.76g of oleic acid and mix evenly, then fill the aluminum can with 14.83g of HFA-113a, seal the aluminum can, and obtain the prepared sample.
Embodiment 2
[0051] (1) Prescription
[0052]
[0053]
[0054] (2) Preparation method
[0055] Mix albuterol and 5.0 mol / L sulfuric acid evenly and pump into the reaction kettle, control the reaction temperature to 15°C, maintain a stirring speed of 900rpm / min, and control the reaction time for 10min. Filter the liquid medicine and dry the fine salbutamol sulfate powder that obtains, see attached through powder electron microscope observation result. figure 1 The results of the particle size data of the pulverized albuterol sulfate measured by laser particle size analyzer are shown in the appendix figure 2 .
[0056] Take 2.84g of salbutamol sulfate, add it to 39.27g of absolute ethanol to dissolve, add 14.76g of oleic acid and mix evenly, then fill the aluminum can with 14.83g of HFA-227, seal the aluminum can, and obtain the prepared sample.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com