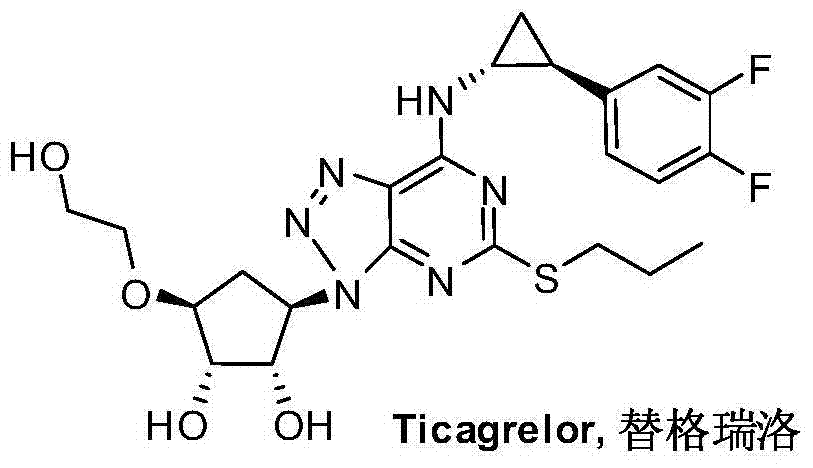
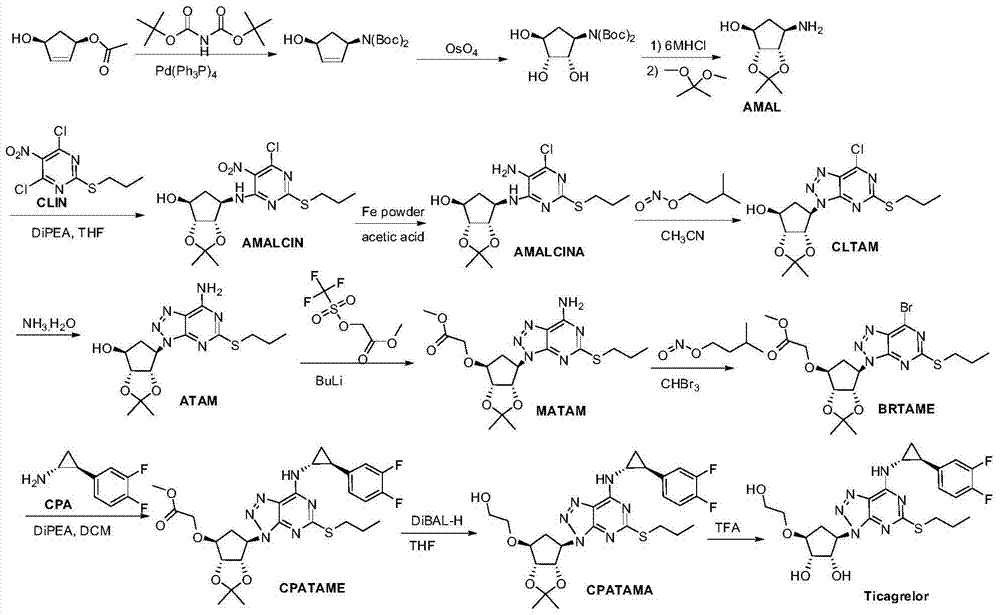
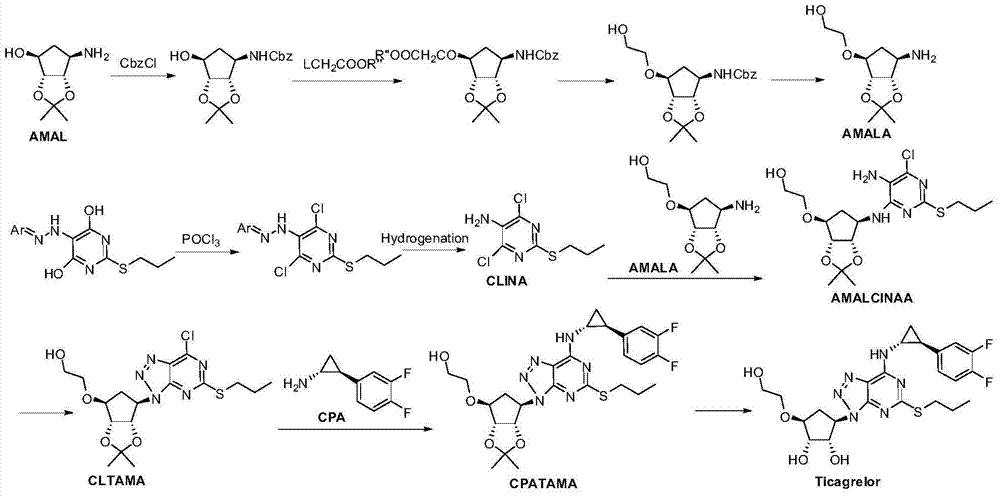
A kind of preparation method of ticagrelor and its new intermediate
A technology for ticagrelor and its compounds, which is applied in the field of intermediate preparation, can solve the problems of expensive starting materials, high cost, and long routes, and achieve easy and effective separation, reduced usage, and cheap and easy-to-obtain starting materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Preparation of 2-propylthio-4-hydroxy-5-nitro-6-chloropyrimidine (compound of formula 2)
[0061]
[0062] Add raw material 1 (30.0g, 129.7mmol) and solvent toluene (300ml) into a 500ml three-neck flask, add DIPEA (18.4g, 142.7mmol) and PCl3 (19.6g, 142.7mmol) under stirring, and stir at room temperature for 2 hours after the addition . Pour the reaction solution into ice water (150ml) to quench, extract with EA (2×150ml), combine the EA layers, wash with water (150ml), wash with saturated brine (150ml), dry over anhydrous sodium sulfate, filter, concentrate and add PE Recrystallization gave light yellow solid 2 (26.2 g, yield 80.9%).
[0063] 1 H NMR (CDCl 3 ,400MHz)δ3.23(2H,J=7.2Hz,t),1.81-1.76(2H,m),1.06(3H,J=7.2Hz,t).
[0064] Compounds of formula 2 can also be prepared using other chlorination reagents.
[0065]
[0066] Add raw material 1 (10.0g, 43.2mmol) and solvent toluene (100ml) into a 250ml three-neck flask, add DIPEA (6.1g, 47.6mmol) and POCl3 (7...
Embodiment 2
[0070] 6-((3aS,4R,6S,6aR)-6-(2-hydroxyethoxy)-2,2-dimethyltetrahydro-3aH-cyclopentyl[d][1,3]dioxo -4-amino)-5-nitro-2-(propylthio)pyrimidine-4-hydroxyl (formula 4a compound) preparation
[0071]
[0072] Add compound 3a (1.0g, 4.6mmol) and solvent ethylene glycol (5ml) into a 100ml single-necked flask, add base TEA (1.4g, 13.8mmol) and compound 2 (1.2g, 4.6mmol) under stirring, and put The temperature of the reaction system was raised to 110° C., and the reaction was stirred for 2 hours. Cool the reaction solution to room temperature, add water (10ml) and EA (10ml) to quench, stir for 10 minutes, separate the organic layer, extract the aqueous layer with EA (10ml), combine the EA layers, wash with water (2×20ml), and saturated salt Washed with water (20ml), concentrated and added PE to precipitate the solid product 4a (1.6g, yield 81.3%), which was directly used in the next reaction.
[0073] 1 H NMR (CDCl 3 ,400MHz)δ11.63(1H,s),10.08(1H,J=7.6Hz,d),4.76-4.68(2H,m),4.58(...
Embodiment 3
[0078] 6-((3aS,4R,6S,6aR)-6-(2-hydroxyethoxy)-2,2-dimethyltetrahydro-3aH-cyclopentyl[d][1,3]dioxo -4-amino)-5-amino-2-(propylthio)pyrimidine-4-hydroxyl (compound of formula 5a)
[0079]
[0080] Add compound 4a (1.0g, 2.3mmol) and solvent methanol (10ml) into a 50ml single-necked bottle, stir to dissolve, add the catalyst Raney nickel (0.3g), and react overnight at room temperature under hydrogen balloon pressure (0.1MPa). After filtration, the filtrate was concentrated to dryness to obtain the reduced product 5a (0.88 g, yield 94.6%), which was directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com