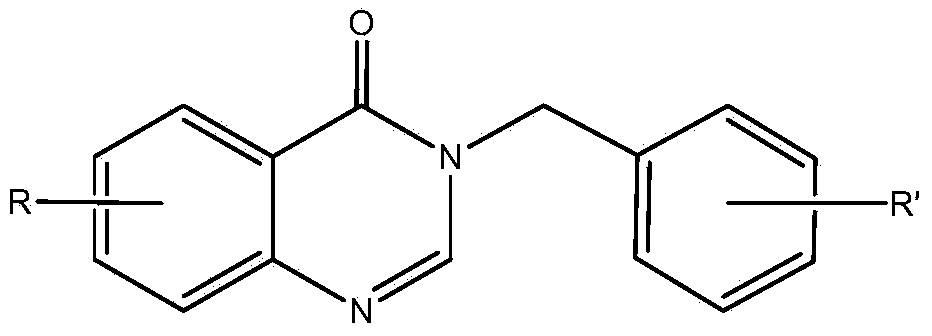
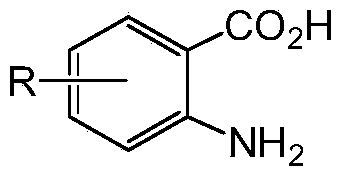
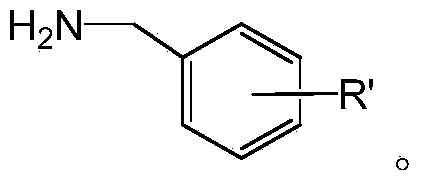
3-benzyl-4-quinazolinone compound as well as synthetic method and applications thereof
A quinazolinone and compound technology, which is applied in the field of 3-benzyl-4-quinazolinone compound and its synthesis, can solve the problems of short action time, weak drug effect and high production cost, and can reduce systolic blood pressure , The synthesis method is easy, and the effect achieved by the synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13-(4
[0055] Preparation of Example 13-(4-methoxybenzyl)-7-fluoro-4-quinazolinone (1)
[0056]Add absolute ethanol (10mL), 2-amino-4-fluorobenzoic acid (0.23g), 4-methoxybenzylamine (0.20g), triethyl orthoformate (0.6mL) into a 50mL round bottom flask , the mixture was stirred and refluxed for 5 h under the protection of nitrogen, the solvent was distilled off under reduced pressure, and the residue was separated by silica gel column chromatography (chloroform:methanol=50:1). 1 H NMR (CDCl 3 ,400MHz),δ:3.79(3H,s,OCH 3 ),5.12(2H,s,Ar-CH 2 ), 6.88(2H,d,J=7.6Hz,Ar-H),7.22(1H,t,J=8.4Hz,Ar-H),7.30(1H,s,Ar-H),7.34(2H,d , J=10.0Hz, Ar-H), 8.11 (1H, s, Ar-H), 8.33 (1H, t, J=8.0Hz, Ar-H).
Embodiment 23-(4
[0057] Example 2 Preparation of 3-(4-methoxybenzyl)-7-chloro-4-quinazolinone (2)
[0058] 2-amino-4-chlorobenzoic acid was used instead of 2-amino-4-fluorobenzoic acid, isopropanol was used instead of absolute ethanol, and the reflux reaction time was 8 hours. The rest of the operations were the same as in Example 1. 1 H NMR (CDCl 3 ,400MHz),δ:3.79(3H,s,OCH 3 ),5.12(2H,s,Ar-CH 2 ),6.88(2H,d,J=7.6Hz,Ar-H),7.31(2H,d,J=7.6Hz,Ar-H),7.45(1H,d,J=8.4Hz,Ar-H), 7.71 (1H, s, Ar-H), 8.28 (2H, d, J=8Hz, Ar-H).
Embodiment 33-(4
[0059] Example 3 Preparation of 3-(4-methoxybenzyl)-6-bromo-4-quinazolinone (3)
[0060] 2-amino-5-bromobenzoic acid was used instead of 2-amino-4-fluorobenzoic acid, 2-methoxyethanol was used instead of ethanol, the reflux reaction time was 3 hours, and the rest of the operations were the same as in Example 1. 1 H NMR (CDCl 3 ,400MHz),δ:3.81(3H,s,OCH 3 ),5.16(2H,s,Ar-CH 2 ),6.90(2H,d,J=8.0Hz,Ar-H),7.33(2H,d,J=8.0Hz,Ar-H),7.62(1H,d,J=8.4Hz,Ar-H), 7.85 (1H, d, J=8.8Hz, Ar-H), 8.16 (1H, s, Ar-H), 8.45 (1H, s, Ar-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com