Antipodal kauri alkane glycoside compounds as well as preparation method and applications thereof
A technology of kauritanes and compounds, which is applied in the field of medicine, can solve the problems of rare research reports and few research reports on the active chemical components of chrysanthemum berries, and achieve broad application prospects and the effect of improving activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
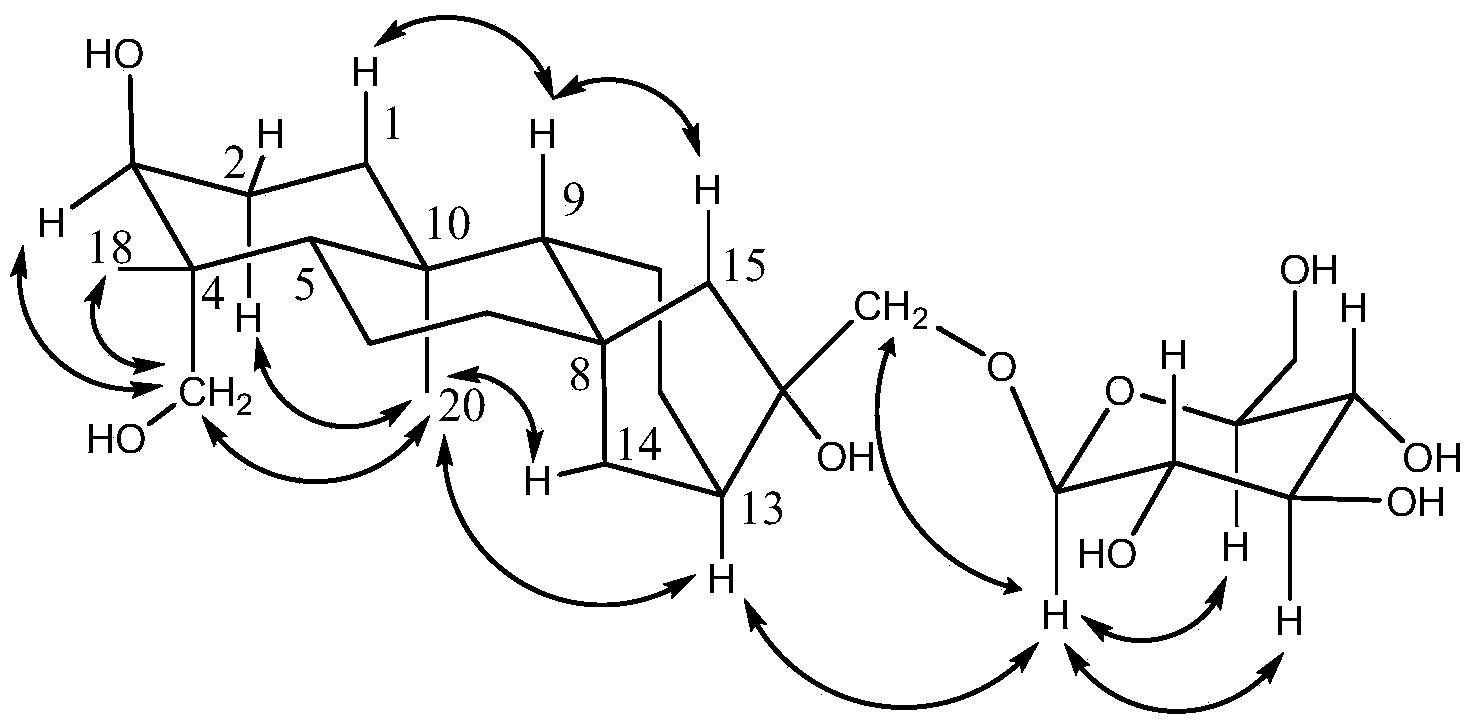
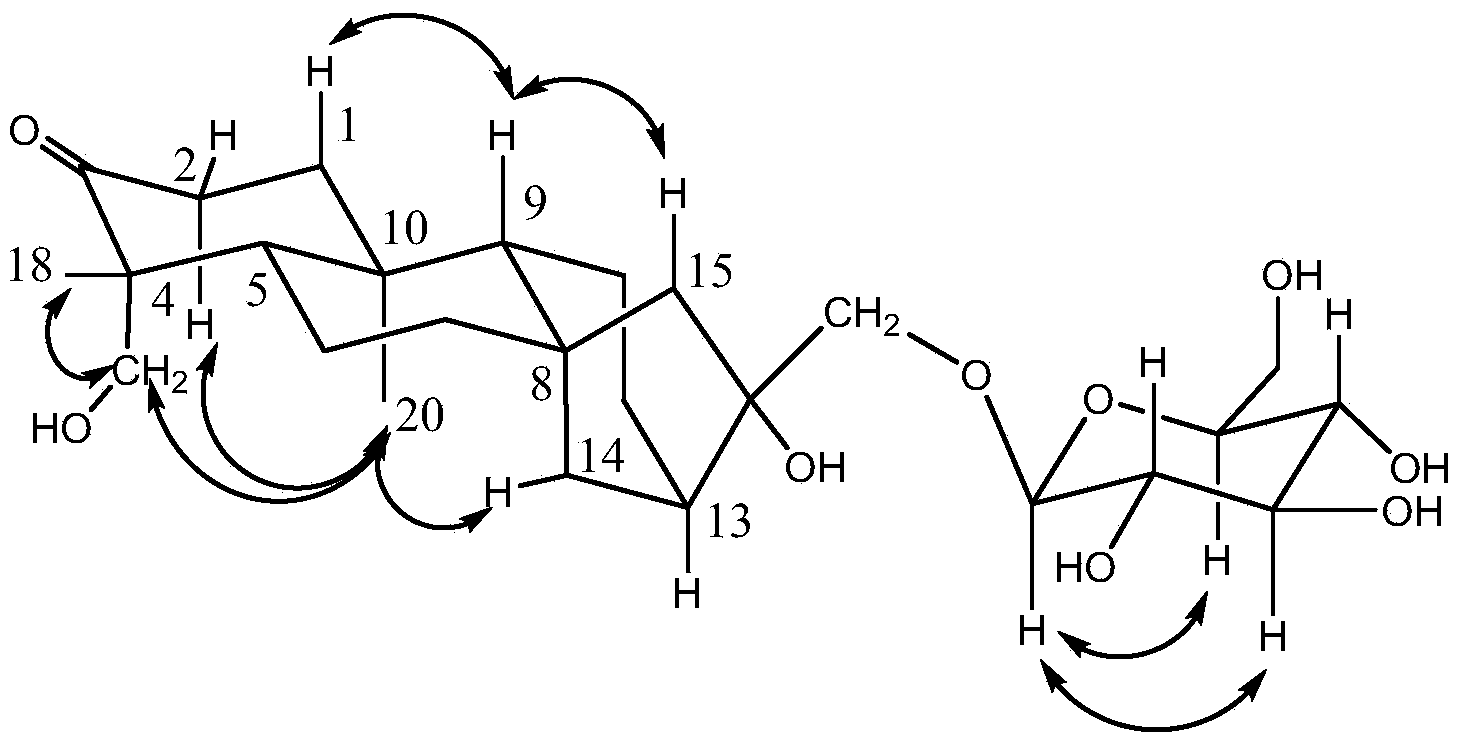
[0020] Example 1. Extraction, separation and identification of ent-kaurene glycoside compounds
[0021] 1. Extraction and separation
[0022] a. Extraction: dry and pulverize the stems and leaves of the strawberry, and divide them into two parts on average, one of which uses ethanol with a concentration of 65% by volume as a solvent, and extracts it by reflux for 3 times, adding an amount equivalent to the stems and leaves of the strawberry each time. 17 times the total weight of solvent extraction for 1 hour, collect the extract; another part uses ethanol with a volume percentage concentration of 65% as a solvent for percolation, and collects the percolation liquid; combines the extraction liquid and the percolation liquid, and recovers ethanol under reduced pressure , to prepare liquid extract;
[0023] b. Extraction: Disperse the liquid extract obtained in step a in water, extract with petroleum ether, water-saturated ethyl acetate, and water-saturated n-butanol successive...
Embodiment 2
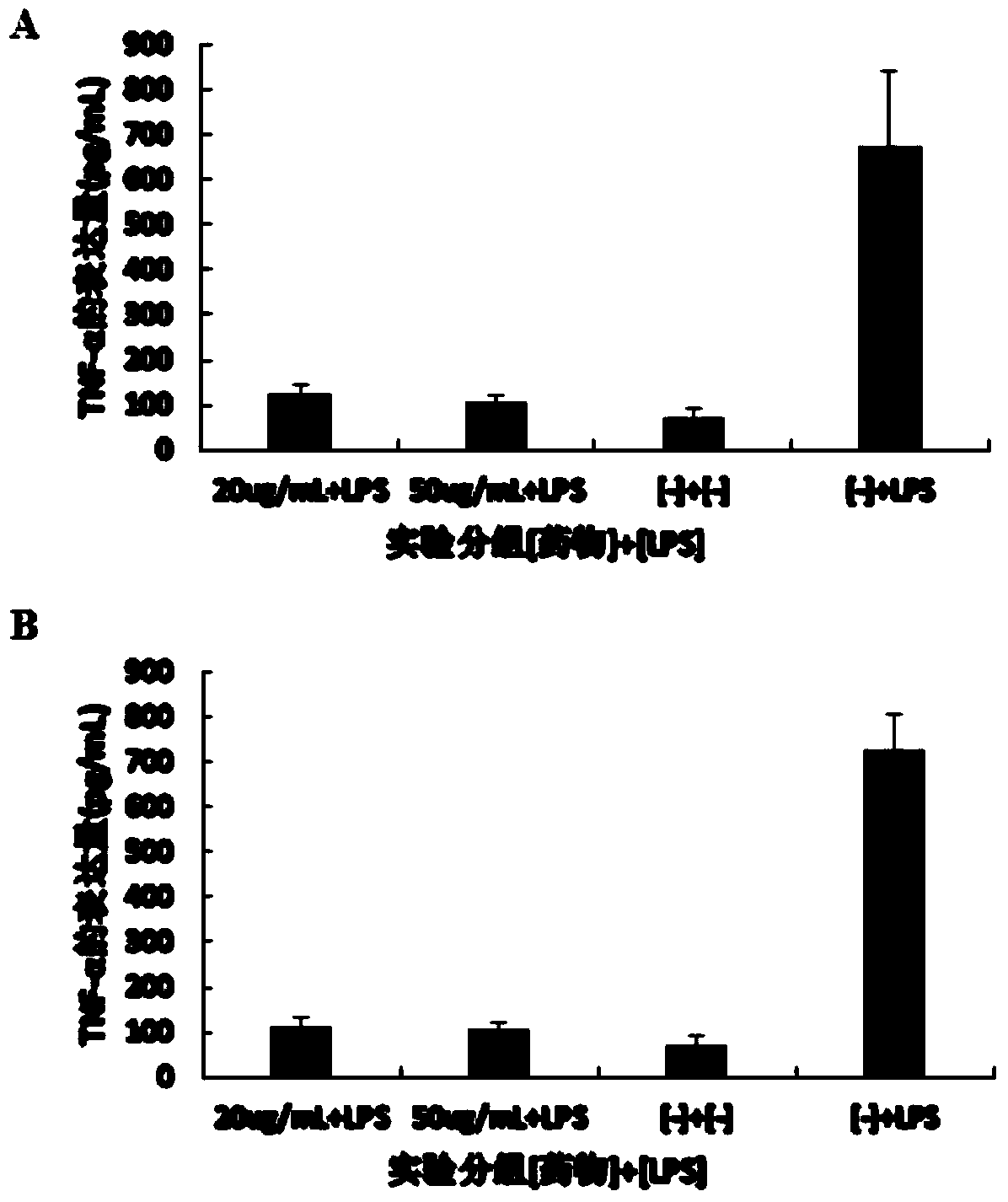
[0036] Example 2. Research on anti-inflammatory activity of ent-kaurene-type glycoside compounds
[0037] Collect mouse peritoneal macrophage RAW264.7 in the logarithmic growth phase, and use 1×10 5cells / mL were inoculated in 96-well cell culture plates, and divided into four experimental groups: simple endotoxin lipopolysaccharide (LPS) stimulation group, low concentration compound treatment group (20 μg / mL), high concentration compound treatment group (50 μg / mL) mL) and the blank control group, with three replicate wells for each group. After the cells adhered to the wall, the low-concentration and high-concentration compound treatment groups were respectively added corresponding concentrations of enantio-kaurane-type glycoside compounds with the structural formula I or II, and incubated for 2 hours. Then, the simple endotoxin LPS stimulation group, low-concentration LPS with a final concentration of 10 pg / mL was added to the treatment group and high-concentration compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com