Synthetic method of azacitidine
A synthesis method and technology of azacitidine are applied in the field of synthesis of azacitidine and can solve the problems of easy emulsification, inconvenience of emulsification, excessive metal tin of crude drug and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A kind of synthetic method of azacitidine of the present embodiment comprises the following steps:
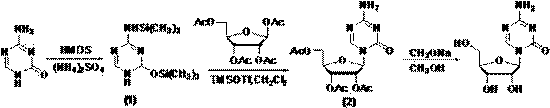
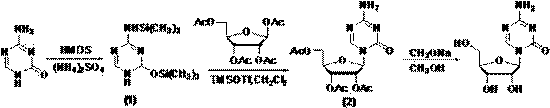
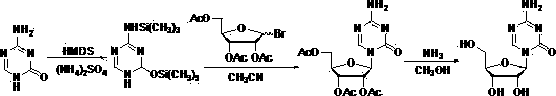
[0031] In step 1, 5-azacytosine and tetraacetyl ribose are used as raw materials, and the compound with the structure of formula (1) is generated after trimethyl silicon protection; wherein, the molar ratio of 5-azacytosine to tetraacetyl ribose is 1:2.5.
[0032] Step 2, compound of formula (1) and 1,2,3,5-tetra-O-acetyl- β -D-ribose is docked into a glycoside of formula (2) under the catalysis of Lewis acid trimethylsilyl trifluoromethanesulfonate (TMSOTf);
[0033] Step 2: Azacitidine is obtained by recrystallization after the alcoholysis of the glycoside with the structure of the above formula (2) to remove the protecting group; in the present invention, the Lewis acid trimethylsilyl trifluoromethanesulfonate (TMSOTf) Instead of tin tetrachloride, its synthetic route is represented by the formula:
[0034]
[0035] In this embodiment, the alcoholysis under alka...
Embodiment 2
[0037] In this embodiment, the molar ratio of 5-azacytosine to tetraacetyl ribose is 1:2.0. Its processing method is identical with embodiment 1.
Embodiment 3
[0039] In this embodiment, the molar ratio of 5-azacytosine to tetraacetyl ribose is 1:3.5. Its processing method is identical with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com