Applications of CTx to preparation of medicaments for diagnosing colorectal cancer
A technology for colorectal cancer and rectal cancer, which can be used in disease diagnosis, biological testing, biological material analysis, etc., and can solve problems such as unreported clinical value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Collection of colorectal cancer patient samples and normal samples at different stages
[0038] From May 2009 to May 2010, we collected tissue and serum samples from 91 patients with colorectal cancer (the patients had been diagnosed with colorectal cancer by histopathological examination, and according to the International Union Against Cancer (UICC) and The TNM (tumor-node-metastasis) staging method jointly developed by the American Cancer Society (AJCC) determined the pathological stage, including 21 patients with stage I, 41 patients with stage II, 22 patients with stage III, and 7 patients with stage IV. laparoscopic surgery), and collected 33 healthy human serum samples without digestive tract diseases and inflammatory diseases. All patients were followed up every 3 months in the first 2 years after surgery, followed up every 6 months in the third year after surgery, and stopped at the 41st month after surgery, and the time points of recurrence or death...
Embodiment 2
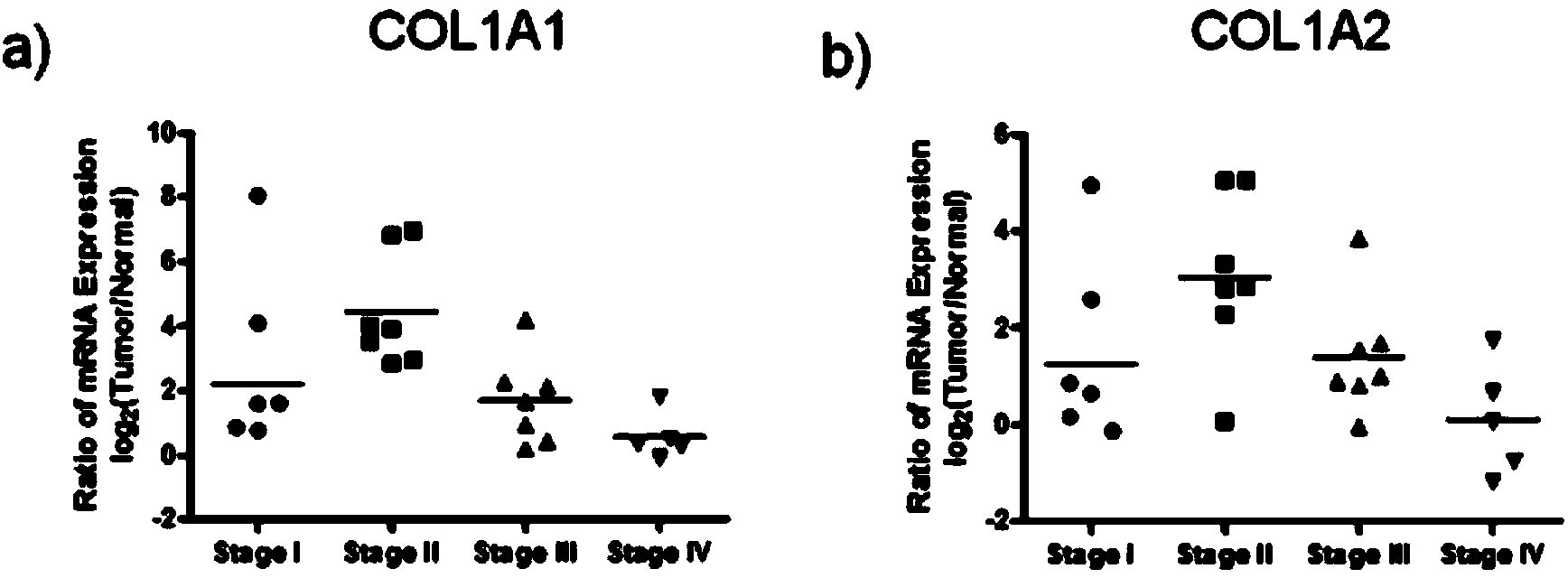
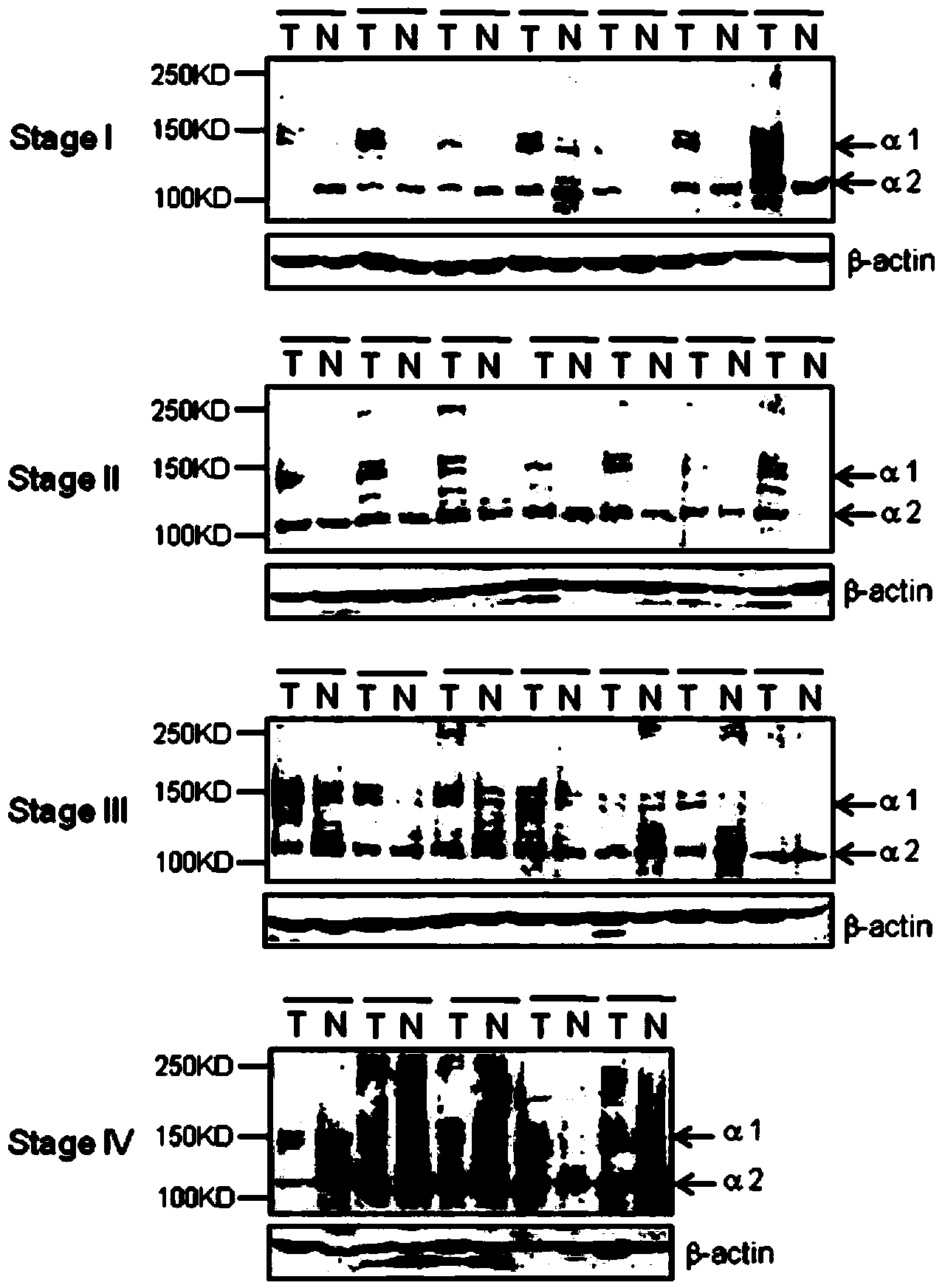
[0042] Example 2: Expression of Type I Collagen at RNA and Protein Levels in Different Stages of Colorectal Cancer Tissues
[0043] 1. Tissue RNA extraction and real-time fluorescent quantitative PCR (Realtime PCR) detection:
[0044] We extracted the total mRNA of tumor tissue and corresponding normal colorectal tissue, and detected the mRNA expression level of type I collagen respectively. The total mRNA of the tissue was extracted with the Trizol kit from Invitrogen, and 20ml of cDNA was synthesized by reverse transcription from 2 mg of RNA using the PrimeScript RT reagent Kit from Takara, and the 7500 real-time fluorescent quantitative PCR instrument from Applied Biosystems and the corresponding SYBR Green PCR were used. The Master Mix kit was used for real-time fluorescent quantitative PCR (2-△△Ct analysis method was used to analyze the expression abundance, and GAPDH was used as an internal reference gene). The primer sequences used in the PCR reaction are shown in Tabl...
Embodiment 3
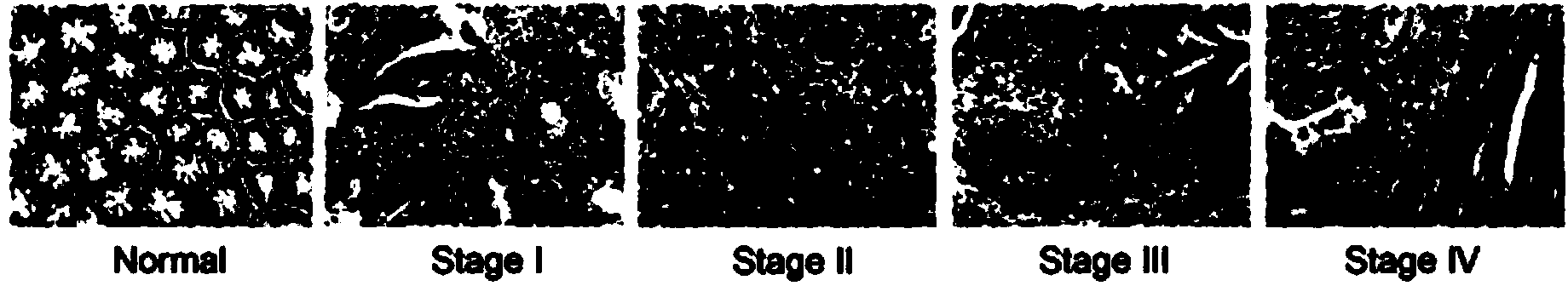
[0059] Example 3: ELISA detection of type I collagen degradation index CTx and random degraded small fragment COL1 in the serum of normal and different stages of colorectal cancer patients:
[0060] 1. ELISA detection kit and method
[0061] The levels of COL1 and CTx in serum can be detected by ELISA using a commercial kit (Uscn Life Science&Technology Co., Houston, TX, USA), and serum CTx can be detected by linear EKAHDGGR peptide (fragment of α1 chain carboxy-terminal peptide of type I collagen). Competitive inhibition ELISA detection of specific monoclonal antibodies, in short, is biotin-labeled synthetic CTx peptides and unlabeled CTx in serum and pre-coated specific CTx antibodies for competitive inhibition reactions, each reaction Add avidin horseradish peroxide into the microwells and incubate. For the specific detection method, refer to the kit instructions. After adding the substrate solution, the color of the reaction mixture was negatively correlated with the conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com