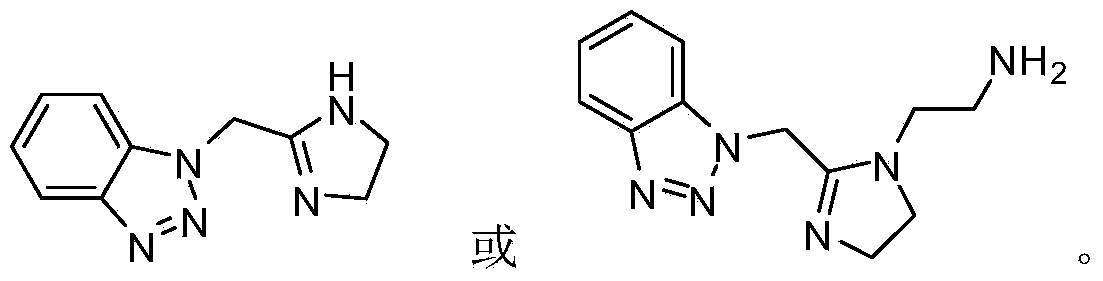
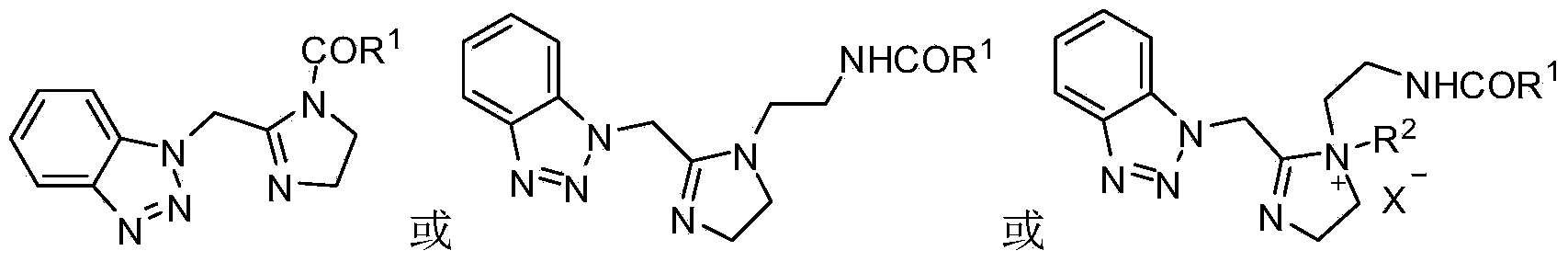
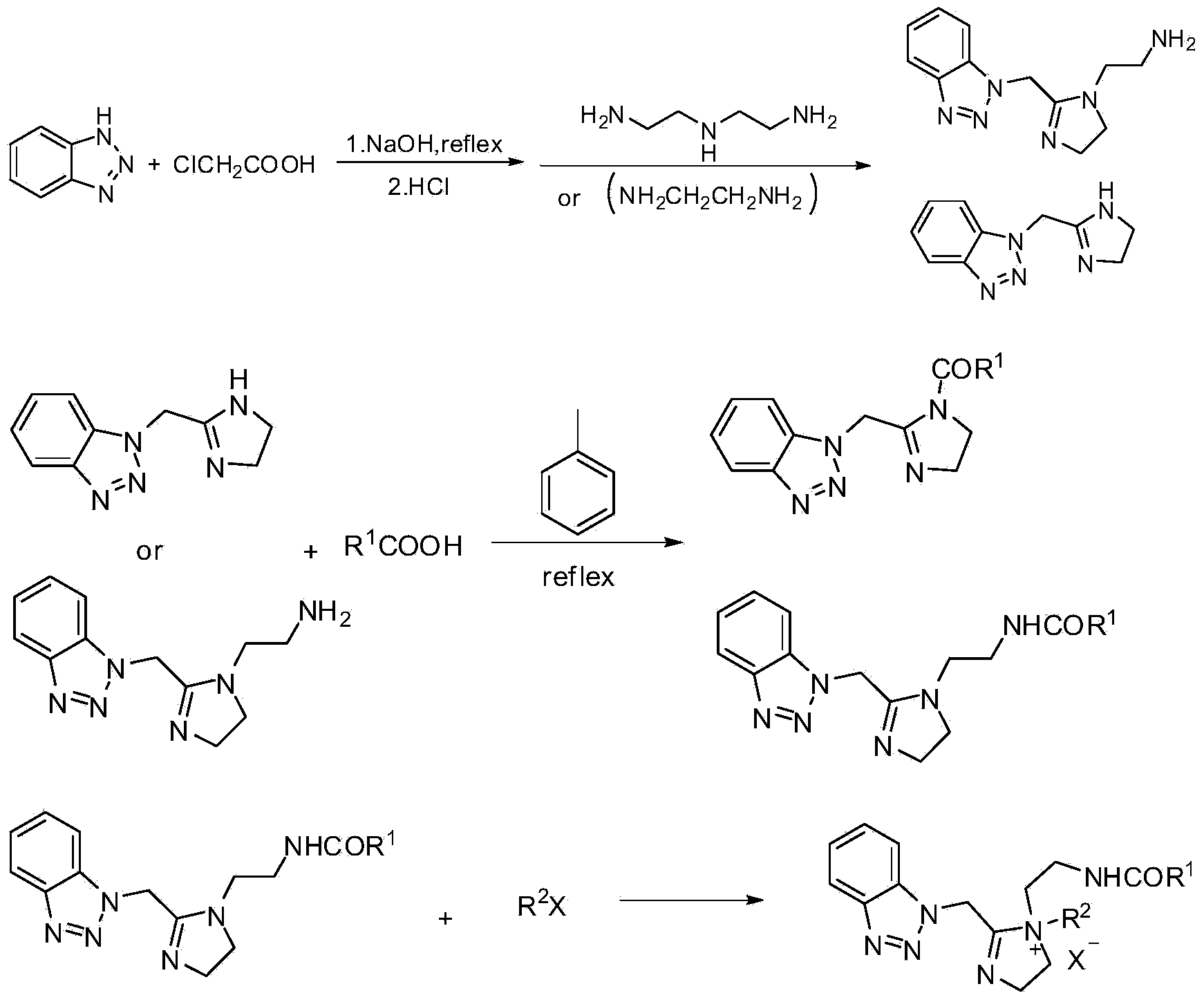
Benzotriazole imidazoline and derivative and synthesis process and application thereof
A technology of benzotriazole imidazoline and benzotriazole, applied in the field of benzotriazole imidazoline, can solve the problems of oil field pipeline corrosion, serious, economic loss and the like, and achieve the effect of increasing corrosion resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 0.1 mol of benzotriazole, 0.21 mol of chloroacetic acid, and 50 mL (6 mol / L) of NaOH solution into a 250 mL round bottom flask and stir for half an hour, then heat up and reflux for 2-6 hours. After the reaction is over, put the flask into ice water and slowly add 60mL of 6mol / L HCl solution dropwise into the flask. At this time, a large amount of white solids can be seen to precipitate. When no solids are precipitated, suction filter and dry the solids for later use. ;
[0028] Put the product of the previous step into a 250ml round bottom flask, add 0.25mol of diethylenetriamine and 50mL (0.4mol) of xylene solution, use a water separator, stir with a magnetic stirrer, and heat with an electric heating mantle. After 2-8 hours, it is found that no water continues to flow out, continue to increase the temperature, and continue to divide the water for about 2-8 hours. After no water continues to flow out, stop the reaction to obtain a brown-yellow benzotriazole imidaz...
Embodiment 2
[0030] Add the benzotriazole imidazoline (0.1mol) obtained in Example 1, 0.24mol of oleic acid, and 20mL (0.19mol) of toluene into a 250mL round-bottomed flask, raise the temperature and separate water for about 2-5 hours to obtain benzotriazole The azole imidazoline derivatives received 70.3 g of the product, and the yield was 92.63%.
[0031] The concrete reaction equation that embodiment 1 and 2 relate to.
[0032]
Embodiment 3
[0034] Add 1 mol of benzotriazole, 2.9 mol of chloroacetic acid, and 70 mL (6 mol / L) of NaOH solution into a 250 mL round bottom flask, stir for half an hour, then heat up and reflux for 2-6 hours. After the reaction was finished, 6mol / L HCl solution (100mL) was slowly added dropwise into the flask. At this time, a large amount of white solids could be seen to precipitate. When no solids were precipitated, suction filtration was performed, and the solids were dried for later use.
[0035] The product from the previous step was placed in a 250mL round bottom flask, 3.1mol of ethylenediamine, 45mL (0.35mol) of xylene solution, stirred with a water separator, a magnetic stirrer, and heated with an electric heating mantle. After 8 hours, it was found that no water continued to flow out. Continue to increase the temperature and continue to separate water for about 2-8 hours. After no water continued to flow out, the reaction was stopped to obtain a brown-yellow benzotriazole imidazo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com