Production process of 4-(hydroxy-(methyl)phosphinyl)-2-oxobutanoic acid
A technology of oxobutyric acid and production process, which is applied in the field of preparation of organic compounds, can solve the problems of discounted weeding effect, difficult storage, easy copolymerization, etc., and achieve the effect of clean production process, low cost and high weeding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
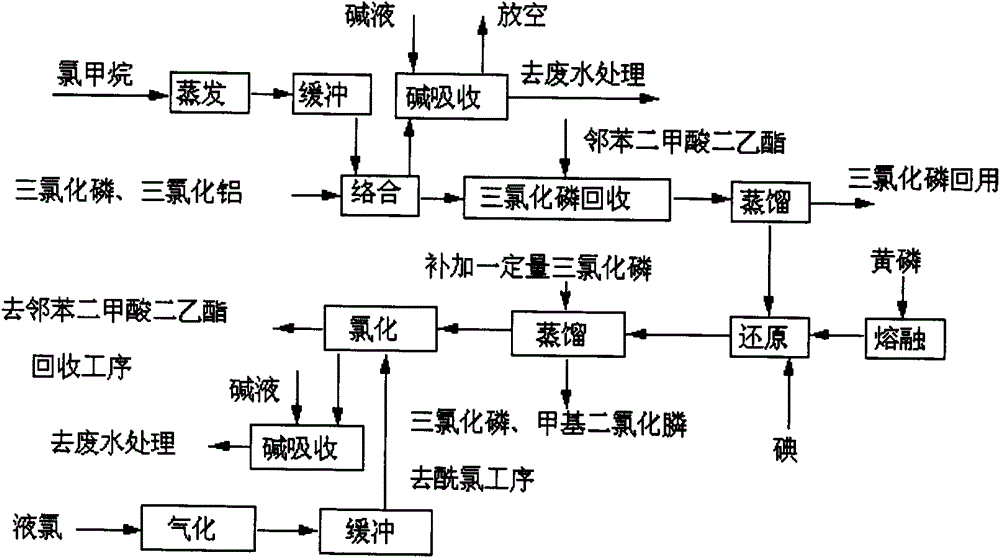
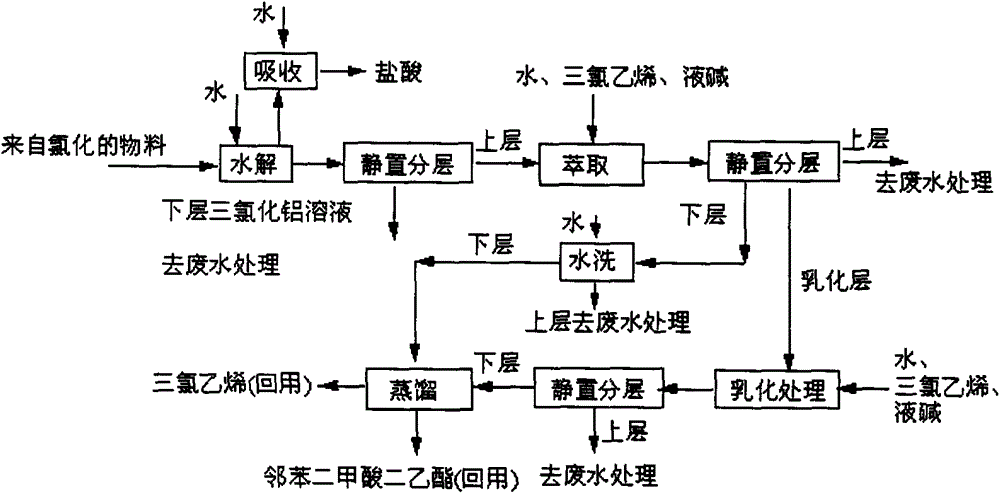
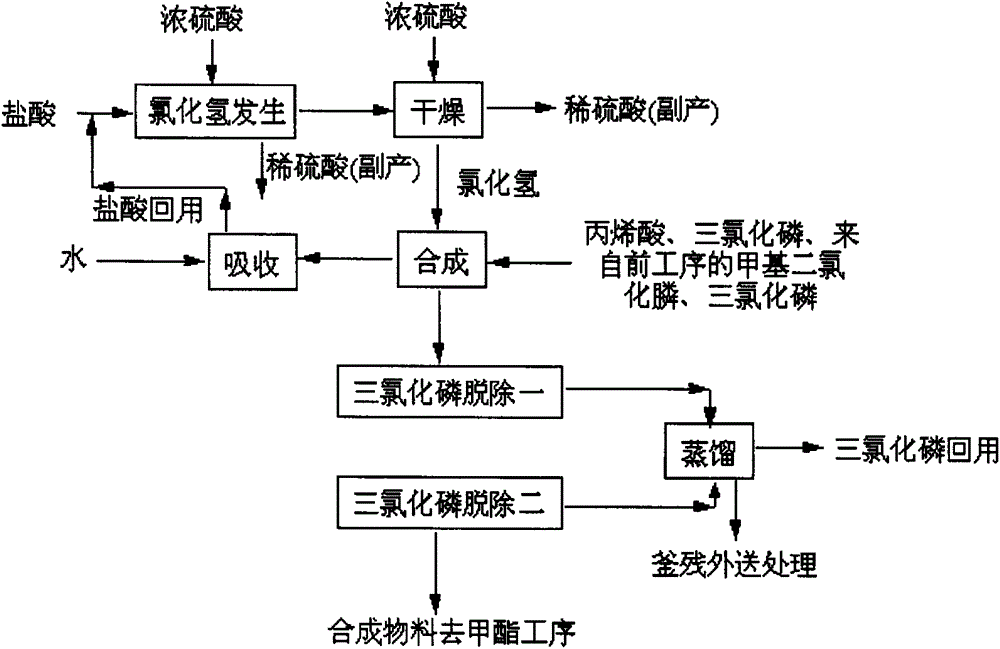
[0061] reference Figure 1 to 6 , A process for producing 4-(hydroxy-(methyl)phosphinyl)-2-oxobutanoic acid, which sequentially includes the following steps:
[0062] (1) Synthesis of methyl phosphine dichloride: add quantitative phosphorus trichloride and aluminum trichloride to the complexing kettle in sequence, stir, heat up (heating by heat transfer oil) to 60℃, and pass in methyl chloride (methyl chloride evaporates) The hot water temperature of the heater is controlled at 40-50°C; the pressure of the methyl chloride buffer tank is about 0.2MPa), the reaction temperature in the complexing kettle is controlled at 70°C, the pressure is 0.2MPa, and the reaction is about 3 hours, stop feeding methyl chloride, and then heat to Keep the temperature at 130°C for 3 hours (control the kettle pressure 3 It is generated into hydrochloric acid and phosphorous acid in the water, and discharged from the exhaust cylinder after being neutralized by the alkali solution, and then transferred ...
Embodiment 2
[0092] reference Figure 1 to 6 , A production process of 4-(hydroxy-(methyl)phosphinyl)-2-oxobutanoic acid, which is different from Example 1 as follows:
[0093] (1) Synthesis of methyl phosphine dichloride: add quantitative phosphorus trichloride and aluminum trichloride to the complexing kettle in turn, stir, heat up (heating by heat transfer oil) to 50℃, and pass in methyl chloride (methyl chloride evaporates) The temperature of the hot water of the heater is controlled at 40-50℃; the pressure of the methyl chloride buffer tank is about 0.2MPa), the reaction temperature in the complexing kettle is controlled at 50℃, the pressure is 0.1MPa, and the reaction is about 5 hours, stop feeding methyl chloride, and then heat to Keep the temperature at 100°C for 3 hours (control the kettle pressure <0.3MPa); after the heat preservation, cool to below 60°C and release the pressure to normal pressure (the gas phase is discharged into the exhaust gas absorption device, and treated with ...
Embodiment 3
[0104] reference Figure 1 to 6 , A production process of 4-(hydroxy-(methyl)phosphinyl)-2-oxobutanoic acid, which is different from Example 1 as follows:
[0105] (1) Synthesis of methyl phosphine dichloride: add quantitative phosphorus trichloride and aluminum trichloride to the complexing kettle in sequence, stir, heat up (heating by heat transfer oil) to 20℃, and pass in methyl chloride (methyl chloride evaporates) The hot water temperature of the heater is controlled at 40-50°C; the pressure of the methyl chloride buffer tank is about 0.2MPa), the reaction temperature in the complexing kettle is controlled at 40°C, the pressure is 0.05MPa, and the reaction is about 8 hours, stop feeding methyl chloride, and then heat to At 60°C, heat preservation for 3 hours (control kettle pressure3 It is generated into hydrochloric acid and phosphorous acid in the water, and discharged from the exhaust cylinder after being neutralized by the alkali solution, and then transferred to the pho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com