Electrolytic bath and method for electrolyzing light rare earth metals or alloys
An electrolytic cell and light rare earth technology, which is applied to cells and other directions, can solve the problems of excessive rare earth metal iron content, bolt entry, etc., and achieve the effects of improving quality, avoiding pollution, and the electrolytic cell and method being simple and easy to implement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
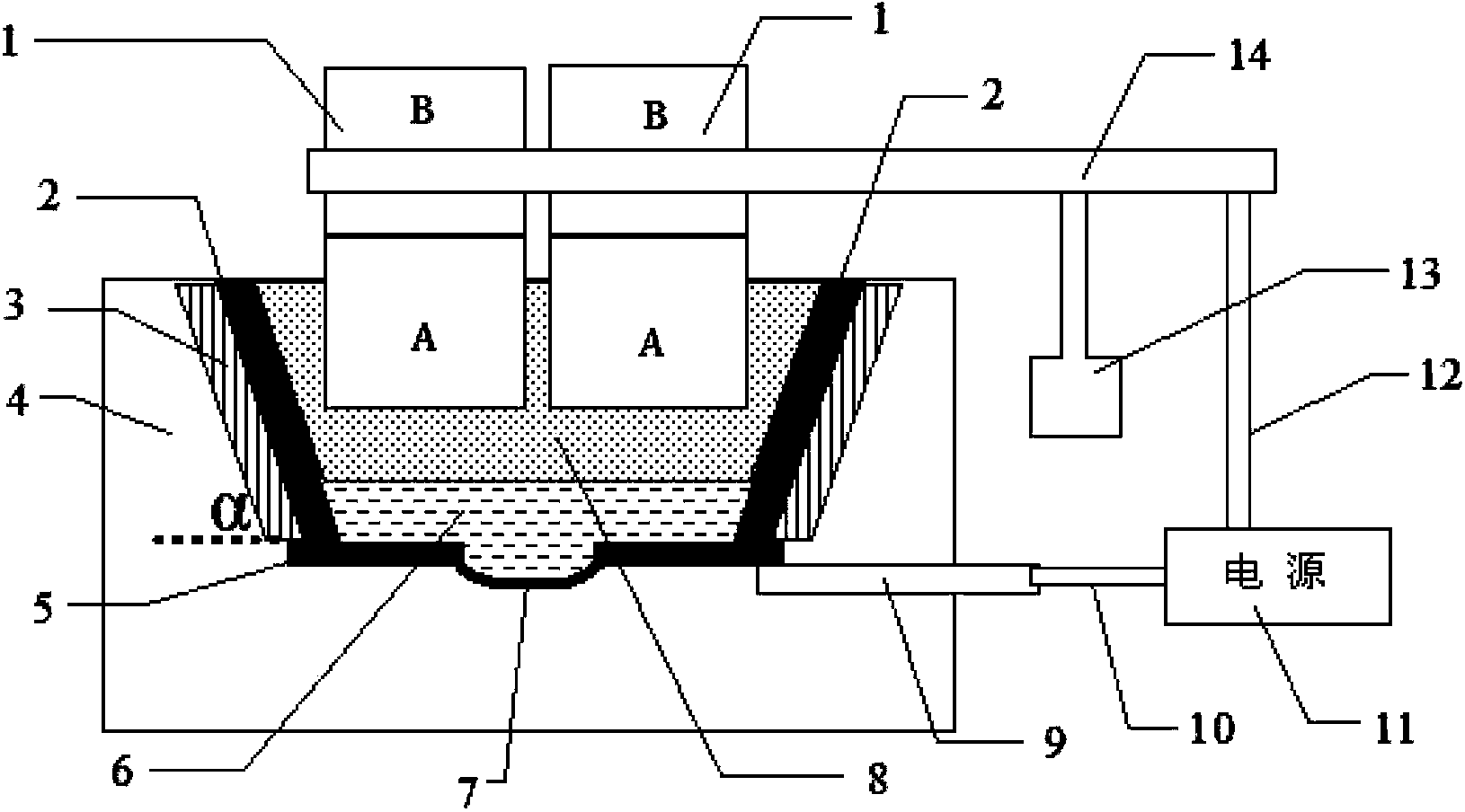
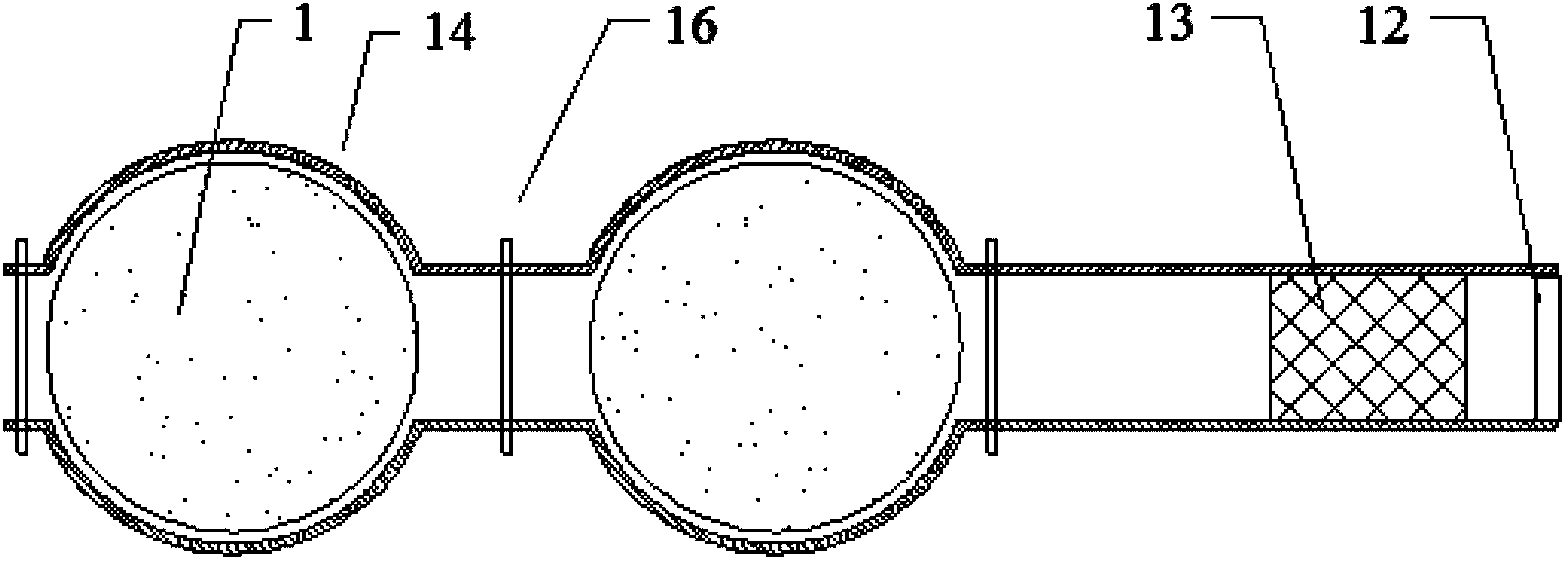
[0052] When starting the furnace, first preheat and dry the electrolytic cell and graphite anode, and inject the melted electrolyte into the electrolytic cell, the electrolyte is LaF 3 A molten solution composed of LiF, where LaF 3 The ratio to LiF is 3 / 1. Start the anode lifting device, so that 6 groups of cylindrical graphite anodes are lowered and immersed in the electrolyte solution for 240mm, and the distance between the cathode and anode is controlled to be 50mm. Turn on the power supply, pass in a current of 2000A, keep the electrolyte in a molten state, add lanthanum oxide, and perform electrolysis. Turn on the cooling and temperature control device in the inner wall, so that the surface temperature of the inner wall is 10°C lower than the solidification temperature of the electrolyte solution, so that the electrolyte solution adheres to the surface of the inner wall and gradually solidifies into a stable crust protective layer, and at the same time, add electrolyte t...
Embodiment 2
[0054] When starting the furnace, first preheat and dry the electrolytic cell and graphite anode, and inject the melted electrolyte into the electrolytic cell, the electrolyte is LaF 3 A molten solution composed of LiF, where LaF 3 The ratio to LiF is 8 / 1. Start the anode lifting device, so that 8 groups of square columnar graphite anodes are lowered and immersed in the electrolyte solution for 10mm, and the distance between the cathode and anode is controlled to be 400mm. Turn on the power supply, pass through a current of 5000A, keep the electrolyte in a molten state, add lanthanum oxide, and perform electrolysis. Turn on the cooling and temperature control device in the inner wall, so that the surface temperature of the inner wall is 100°C lower than the solidification temperature of the electrolyte solution, so that the electrolyte solution adheres to the surface of the inner wall and gradually solidifies into a stable crust protective layer, and at the same time, add ele...
Embodiment 3
[0056] When starting the furnace, first preheat and dry the electrolytic cell and graphite anode, and inject the melted electrolyte into the electrolytic cell, the electrolyte is LaF 3 A molten solution composed of LiF, where LaF 3 The ratio to LiF is 6 / 1. Start the anode lifting device, so that 8 groups of cylindrical graphite anodes are lowered and immersed in the electrolyte solution for 120mm, and the distance between the cathode and anode is controlled to be 100mm. Turn on the power supply, feed a current of 4000A to keep the electrolyte in a molten state, add lanthanum oxide, and perform electrolysis. Turn on the cooling and temperature control device in the inner wall, so that the surface temperature of the inner wall is 20°C lower than the solidification temperature of the electrolyte solution, so that the electrolyte solution adheres to the surface of the inner wall and gradually solidifies into a stable crust protection layer, and at the same time, add electrolyte t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com