Pulmonary delivery of 17-hydroxyprogesterone caproate (17-HPC)
A technology of 17-HPC and progesterone, which is applied in powder delivery, aerosol delivery, nebulizer for treatment, etc., can solve the problem of not improving enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
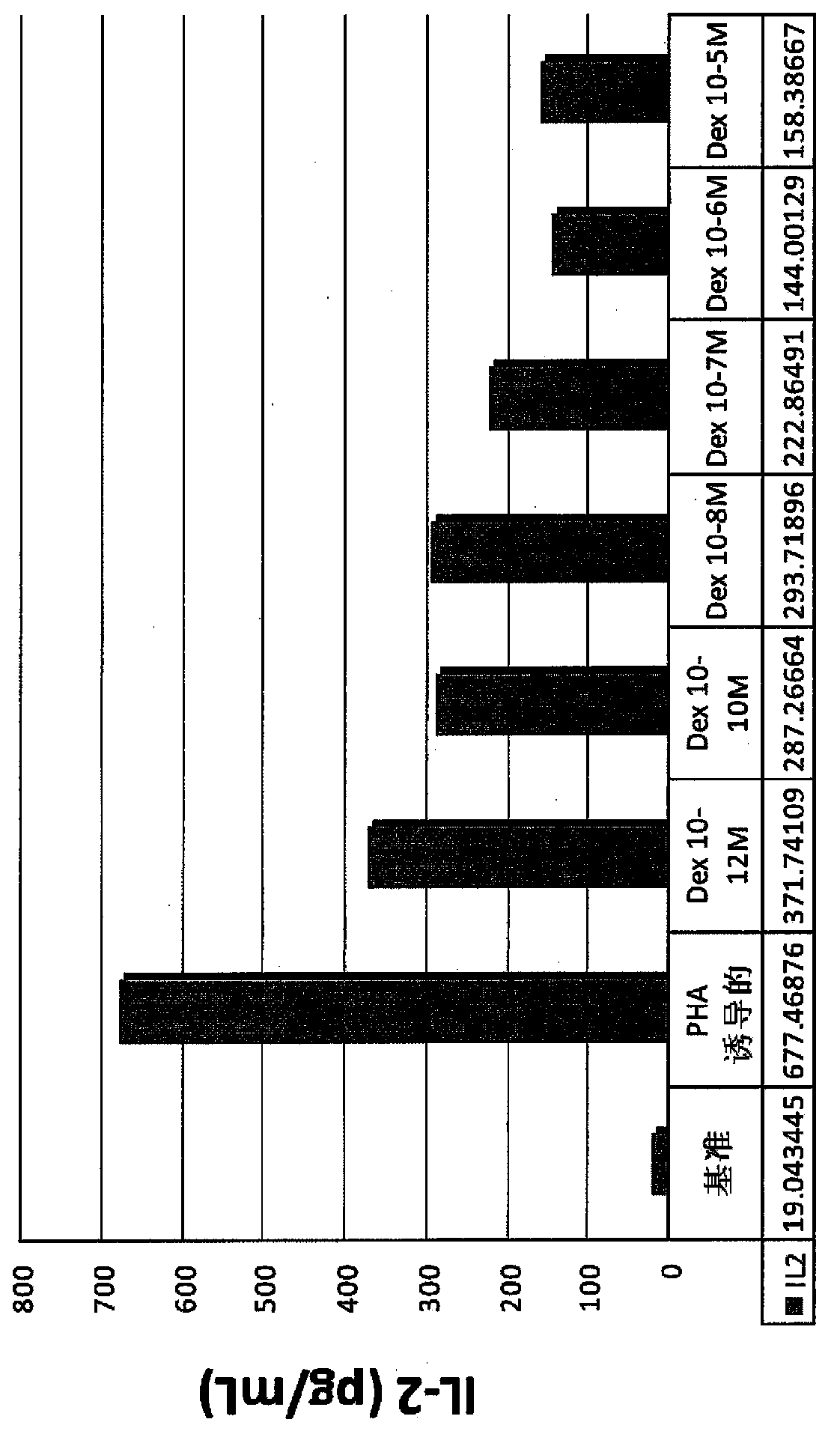
[0131] Example 1. Addition of IL-2 and IL-4 reduces steroid sensitivity or induces steroid resistance in male smokers
[0132] IL-2 / 4-induced steroid resistance in PBMCs, a well-known research model, was used to evaluate potential modulators of steroid resistance and sensitivity. PBMCs were collected from healthy smokers. Corticosteroid insensitivity or tolerance was induced by addition of IL-2 and IL-4 in peripheral blood mononuclear cells (PBMC) from healthy male smokers (n=11). PBMC (10 6 cells / ml) were cultured in 96-well plates for 48 hours, and then exposed to serial dilutions of dexamethasone (10 -10 M, 10 -8 M to 10 -6 M) for 1 hour, then at 37°C, 5% CO 2 Stimulated with PHA (15 μg / ml) for 24 hours. IL-2 levels were quantified using ELISA. The percent inhibition of PHA-induced IL-2 production was calculated, % inhibition = 1 - (IL-2 with dexamethasone / IL-2 without dexamethasone).
[0133] figure 2 The results in showed that the addition of IL-2 and IL-4 sig...
Embodiment 2
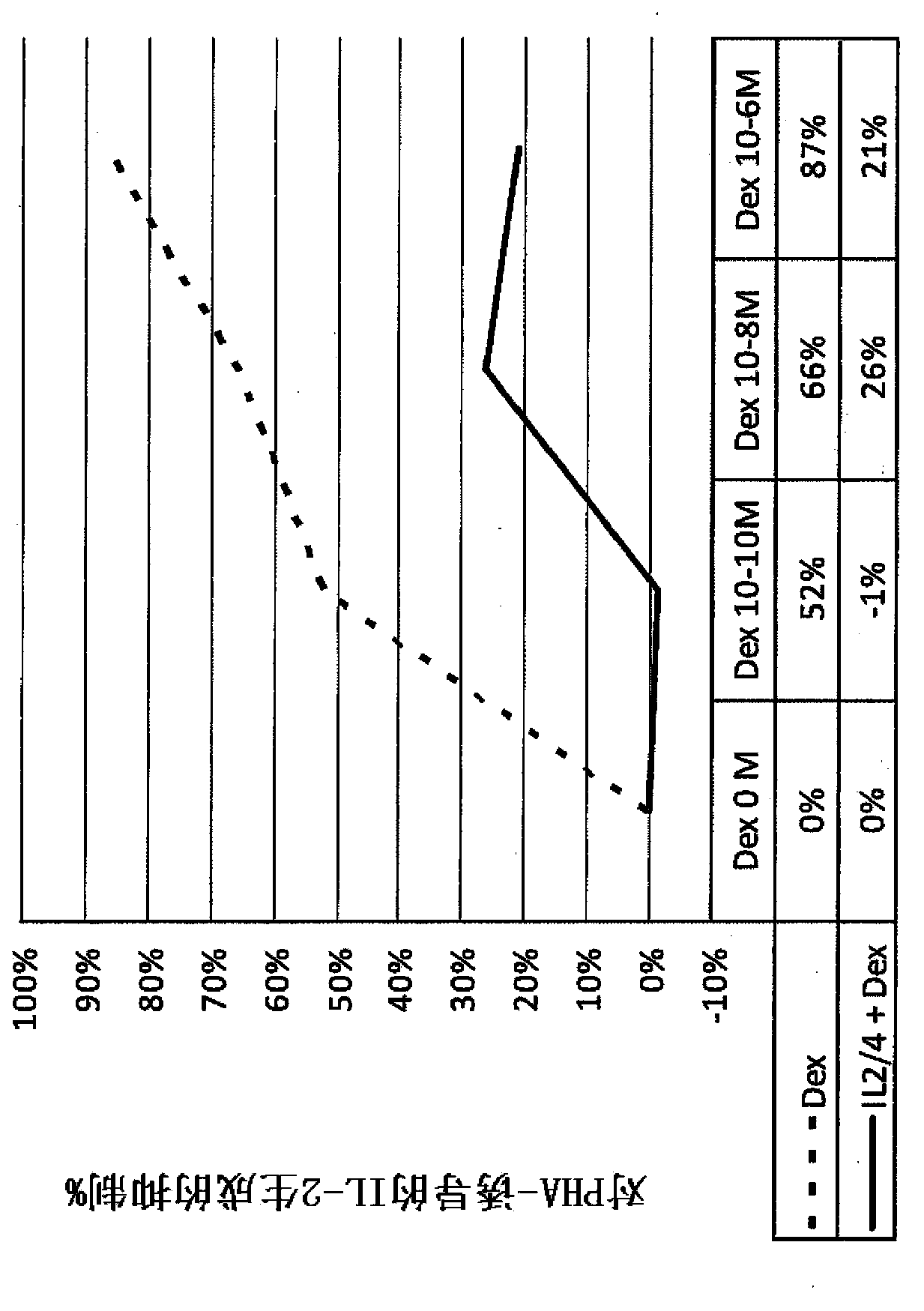
[0134] Example 2. Progestins increase corticosteroid sensitivity or reverse corticosteroid resistance in male smokers.
[0135] Corticosteroid insensitivity or tolerance can be reversed pharmacologically. We investigated the role of progestogens, currently of unknown function, in reversing steroid resistance and tested the progestogenic drug 17α-hydroxyprogesterone caproate in peripheral blood mononuclear cells (PBMCs) from healthy male smokers. Effects of ketone (17HPC), medroxyprogesterone acetate (MPA) and natural progesterone (P4) on enhancing glucocorticoid sensitivity.
[0136] PBMC (10 6 cells / ml) were cultured in 96-well plates for 48 hours, followed by 17HPC (10 -10 M, 10-7 M and 10 -5 M or P4 or MPA (10 -10 M, 10 -8 M and 10 -5 M) Stimulate for 12 hours, and then in 37 ℃, 5% CO 2 , with or without low-dose and high-dose dexamethasone (10 -10 M and 10 -6 M) exposure for 1 hour followed by 24 hours exposure to PHA (15 μg / ml) (n=11 for the combination of 17HP...
Embodiment 3
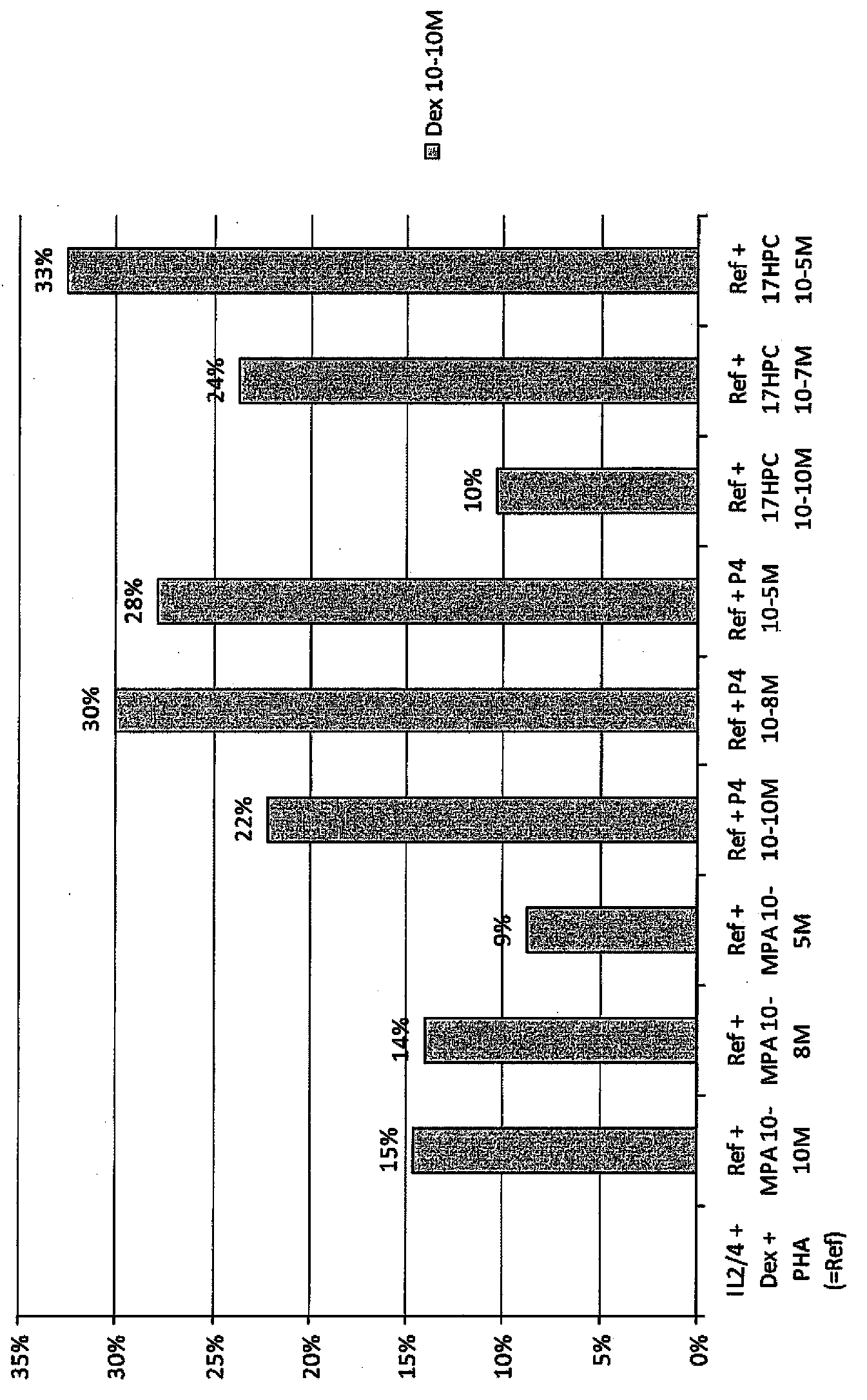
[0140] Example 3. 17HPC reverses corticosteroid tolerance in male smokers
[0141] PBMC (10 6 cells / ml) were cultured in 96-well plates for 48 hours, followed by 17HPC (10 -10 M, 10 -7 M and 10 -5 M) Stimulate for 12 hours, and then in 37 ℃, 5% CO 2 , with or without three doses of dexamethasone (10 -10 M, 10 -8 M and 10 -6 M) exposure for 1 hour followed by 24 hours exposure to PHA (15 μg / ml). IL-2 levels were quantified using ELISA.
[0142] Figure 5 It was shown that addition of IL-2 and IL-4 significantly reduced steroid sensitivity at all three dexamethasone concentrations. Increased dexamethasone inhibition of PHA-induced IL-2 release was obtained by adding 17HPC. In a dose-response plot, 17HPC reversed glucocorticoid insensitivity. Thus, 17HPC restored corticosteroid sensitivity. For example, in the presence of dexamethasone 10 -10 M but without 17HPC, the PHA-induced IL-2 level was 2364pg / ml, vs. -10 M, 10 -7 M and 10 -5 After M, significantly increa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com