Pharmaceutical composition for preventing or treating irritable bowel syndrome
A technique for irritable bowel syndrome and its composition, which is applied in the field of medicine, can solve problems such as the prevention or treatment of irritable bowel syndrome that have not been reported, and achieve the effects of facilitating quality control, simple production process, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
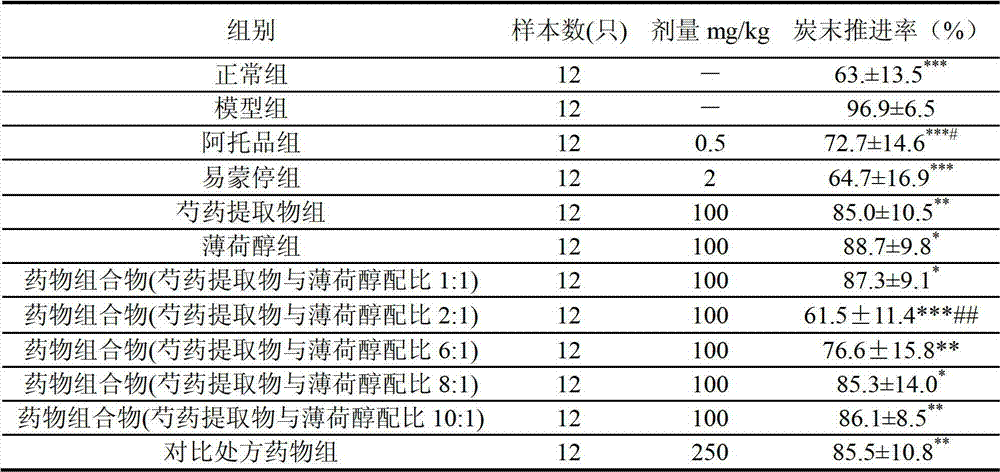
[0018] Embodiment 1 The pharmacodynamics comparative experiment of the pharmaceutical composition of the present invention and the pharmaceutical composition described in CN1660259A
[0019] 1. Experimental materials
[0020] KM mice, weighing 18-22 g, normal grade; animal license number: SCXK (Sichuan) 2004-15, provided by Sichuan Academy of Medical Sciences.
[0021] Peony extract (prepared according to Example 13).
[0022] Contrast prescription drug group: crude drug Radix Paeoniae Alba: Peppermint oil is 130:1, (wherein Radix Paeoniae Alba extraction process prepares Radix Paeoniae Alba extract according to Example 13); Peppermint oil is: batch number 060113 (menthol purity: 47.2%, Xuchang, Henan Huaren Pharmaceutical Co., Ltd.). The above-mentioned raw materials (the Radix Paeoniae Alba extract prepared from 130 parts of Radix Paeoniae Alba and 1 part of peppermint oil) are uniformly mixed to obtain the product.
[0023] Pharmaceutical composition containing peony ext...
Embodiment 2
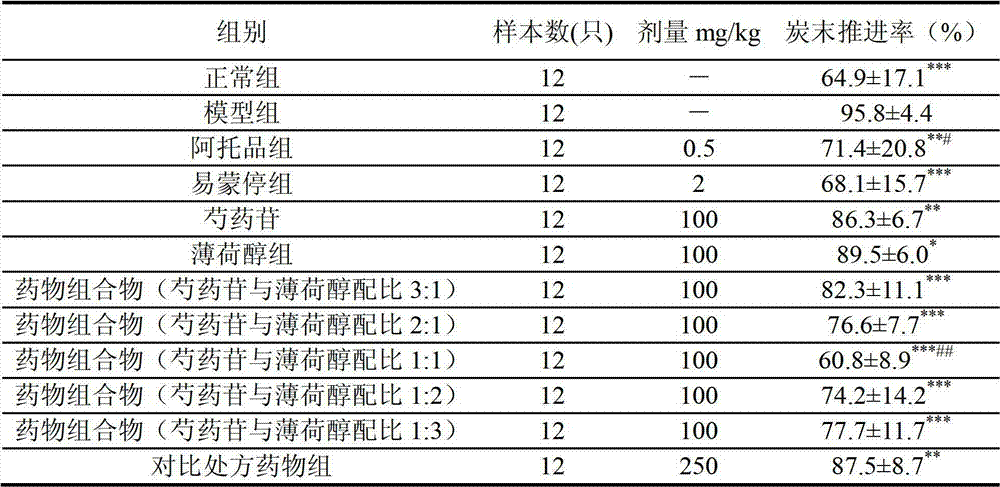
[0032] Embodiment two The pharmaceutical composition of the present invention is compared with the pharmacodynamics experiment of the pharmaceutical composition described in CN1660259A
[0033] 1. Experimental materials
[0034] KM mice, weighing 18-22 g, normal grade; animal license number: SCXK (Sichuan) 2004-15, provided by Sichuan Academy of Medical Sciences.
[0035] Paeoniflorin: batch number 061026, purity 90.5% (refer to the content determination method under "Paeoniae Alba" in the 2005 edition of "Chinese Pharmacopoeia"). Preparation method: after peony is crushed, decoct with 8 times the amount of water for 3 times, each time for 1 hour, the extract is absorbed by D101 macroporous resin, eluted with 50% ethanol, recovered ethanol, concentrated and dried to obtain the extract. The pure paeoniflorin was separated by normal pressure column chromatography.
[0036] Menthol: batch number 060513, purity 99.8%. Produced by Guangzhou Pharmaceutical Chemicals.
[0037] Co...
Embodiment 3
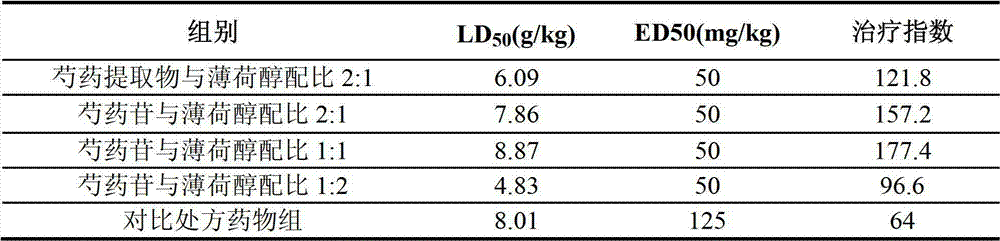
[0046] Example 3 Acute Toxicity Comparison of the Pharmaceutical Composition of the Present Invention and the Comparative Prescription (CN1660259A)
[0047] 1. Experimental materials
[0048] Paeoniae officinalis extract: Same as "Peoniae officinalis extract" in Example 1.
[0049] Menthol: Menthol: batch number 060513, purity 99.8%, produced by Guangzhou Pharmaceutical Chemicals.
[0050] Paeoniflorin: the same as "Paeoniflorin" in Example 2.
[0051] Comparative prescription drug group: prepared according to the comparative prescription drug group in Example 1.
[0052] 2. Experimental method
[0053] Pharmaceutical composition group LD with the ratio of Paeoniae officinalis extract and menthol at a ratio of 2:1 50 Value determination: According to the preliminary test results, the total lethal dose (Dm) of the drug group is 10g / kg, and the zero lethal dose (Dn) is 4g / kg. Take 50 mice and divide them into 5 groups at random, 10 in each group, half male and half male. St...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com