Photochromic niobium pentoxide and preparation method thereof
A niobium pentoxide and photochromic technology, applied in the field of photochromic materials and their preparation, can solve the problem that the photochromic performance of niobium pentoxide has a great influence on the preparation process, and the process conditions of niobium pentoxide have not been discussed in depth. problem, to achieve the effect of good reversible repeatability, fast response and recovery speed, and process safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
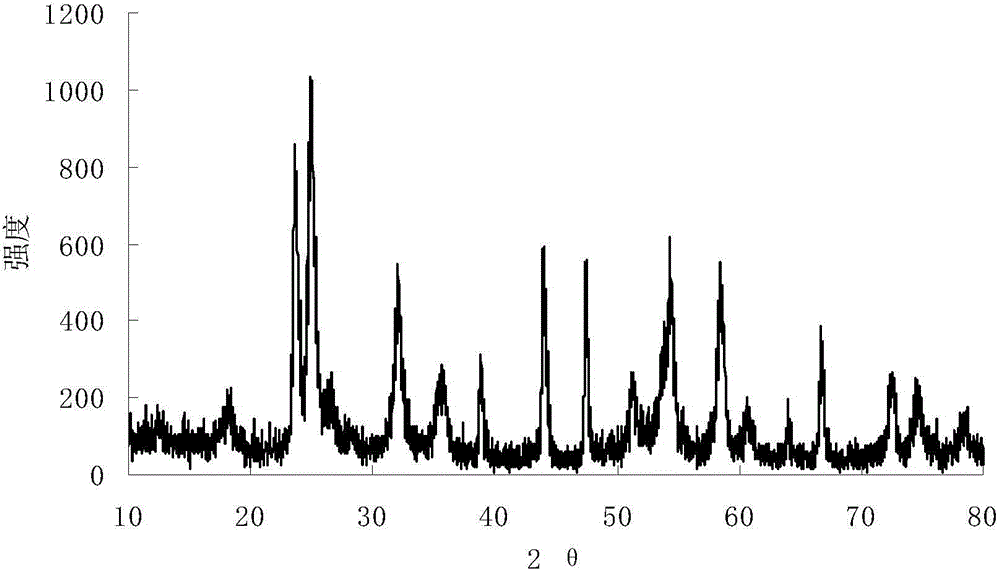
Embodiment 1
[0015] 1) Prepare ammonium oxalate solution: Take 20ml of deionized water and add it to a beaker, put the beaker into a water bath, heat at 60°C, weigh 2.48g of ammonium oxalate into deionized water, stir mechanically until the solution is clear, then make oxalic acid ammonium solution.
[0016] 2) Preparation of ammonium niobium oxalate solution: Weigh 3.76 g of niobium pentafluoride and add it to the ammonium oxalate solution prepared in step 1), to obtain ammonium niobium oxalate solution.
[0017] 3) Add ammonia water dropwise to the ammonium niobium oxalate solution prepared in step 2) using a rubber dropper to start to produce white precipitates. When the pH value of the solution is 11, the reaction is complete. Put the fully reacted solution in an oven to age and precipitate. The heating temperature is 80° C., and the aging time is 12 hours.
[0018] 4) Filter the white precipitate aged in step 3), and wash it repeatedly with ultrapure water to remove fluoride ions. T...
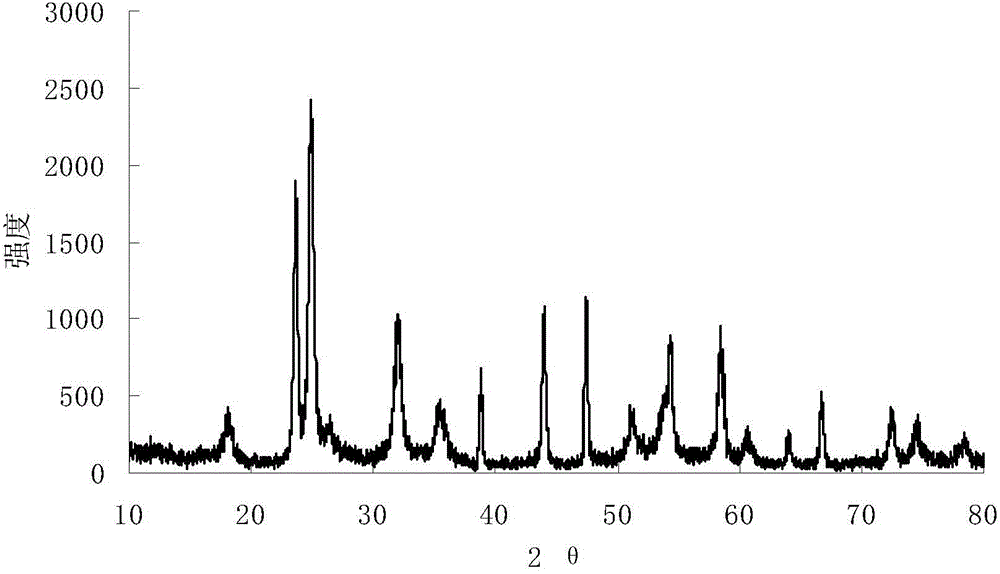
Embodiment 2
[0022] 1) Prepare ammonium oxalate solution: Take 30ml of deionized water and add it to a beaker, put the beaker into a water bath, heat at 70°C, weigh 4.96g of ammonium oxalate into deionized water, and stir mechanically until the solution is clear to make oxalic acid ammonium solution.
[0023] 2) Preparation of ammonium niobium oxalate solution: Weigh 7.52 g of niobium pentafluoride and add it to the ammonium oxalate solution prepared in step 1), to obtain ammonium niobium oxalate solution.
[0024] 3) Add ammonia water dropwise to the ammonium niobium oxalate solution prepared in step 2) using a rubber dropper to start to produce white precipitates. When the pH value of the solution is 11, the reaction is complete. Put the fully reacted solution in an oven to age and precipitate. The heating temperature was 80° C., and the aging time was 16 hours.
[0025] 4) Filter the white precipitate aged in step 3), and wash it repeatedly with ultrapure water to remove fluoride ions....
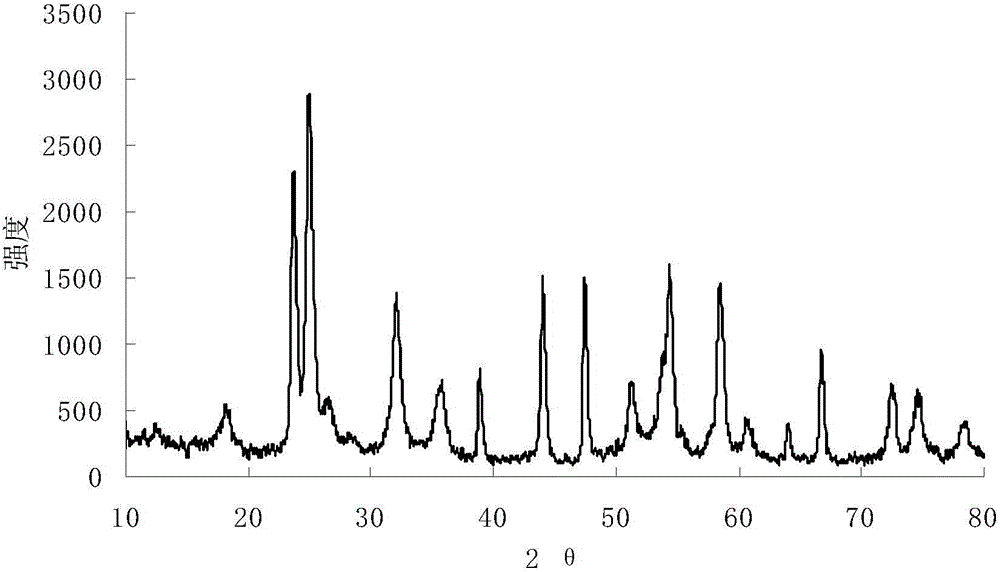
Embodiment 3
[0029] 1) Prepare ammonium oxalate solution: Take 40ml of deionized water and add it to a beaker, put the beaker into a water bath, heat at 80°C, weigh 8.07g of ammonium oxalate into deionized water, stir mechanically until the solution is clear, then make oxalic acid ammonium solution.
[0030] 2) Preparation of ammonium niobium oxalate solution: Weigh 12.22 g of niobium pentafluoride and add it to the ammonium oxalate solution prepared in step 1), to obtain ammonium niobium oxalate solution.
[0031] 3) Add ammonia water dropwise to the ammonium niobium oxalate solution prepared in step 2) using a rubber dropper to start to produce white precipitates. When the pH value of the solution is 11, the reaction is complete. Put the fully reacted solution in an oven to age and precipitate. The heating temperature is 80° C., and the aging time is 18 hours.
[0032] 4) Filter the white precipitate aged in step 3), and wash it repeatedly with ultrapure water to remove fluoride ions. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com