A kind of preparation method and application of photochromic chain transfer agent
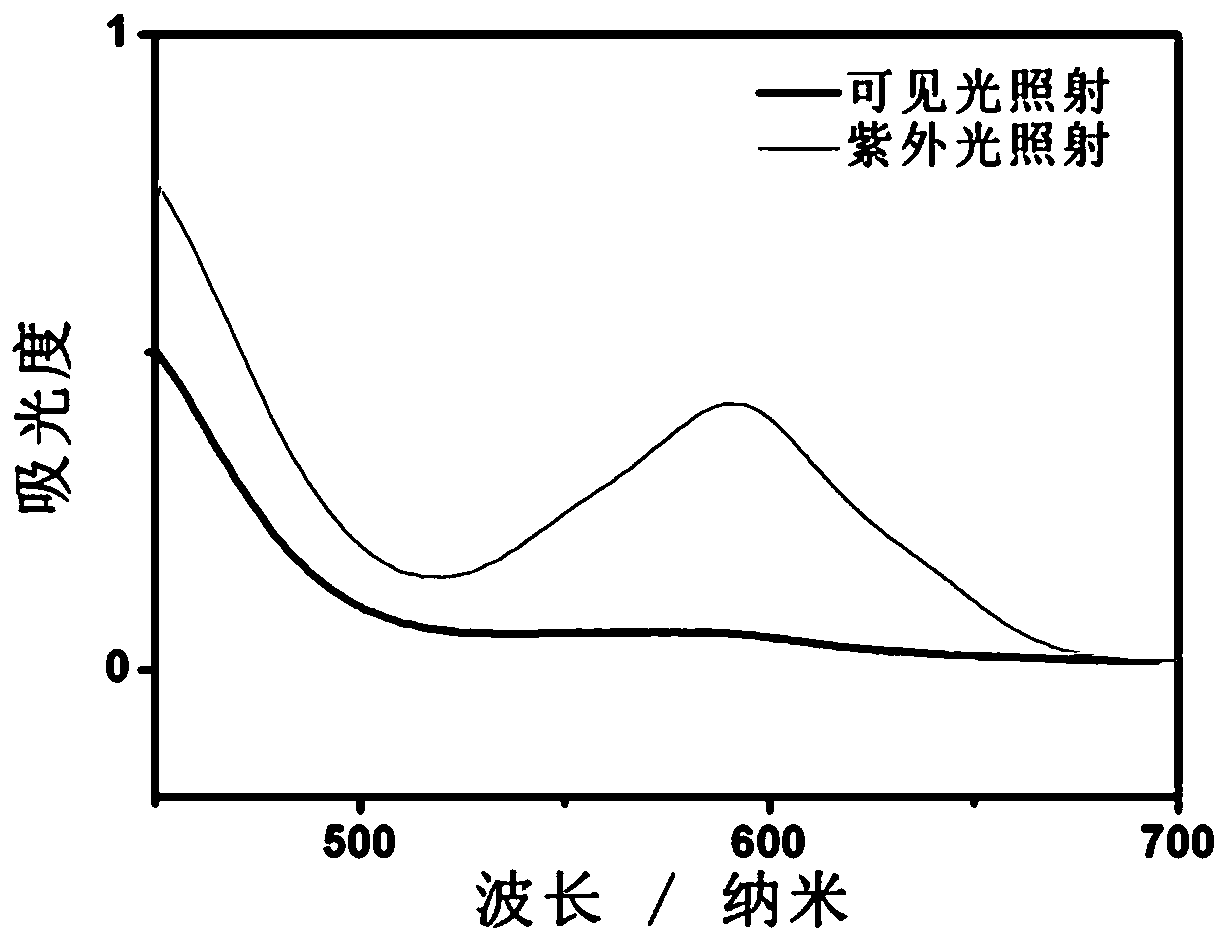
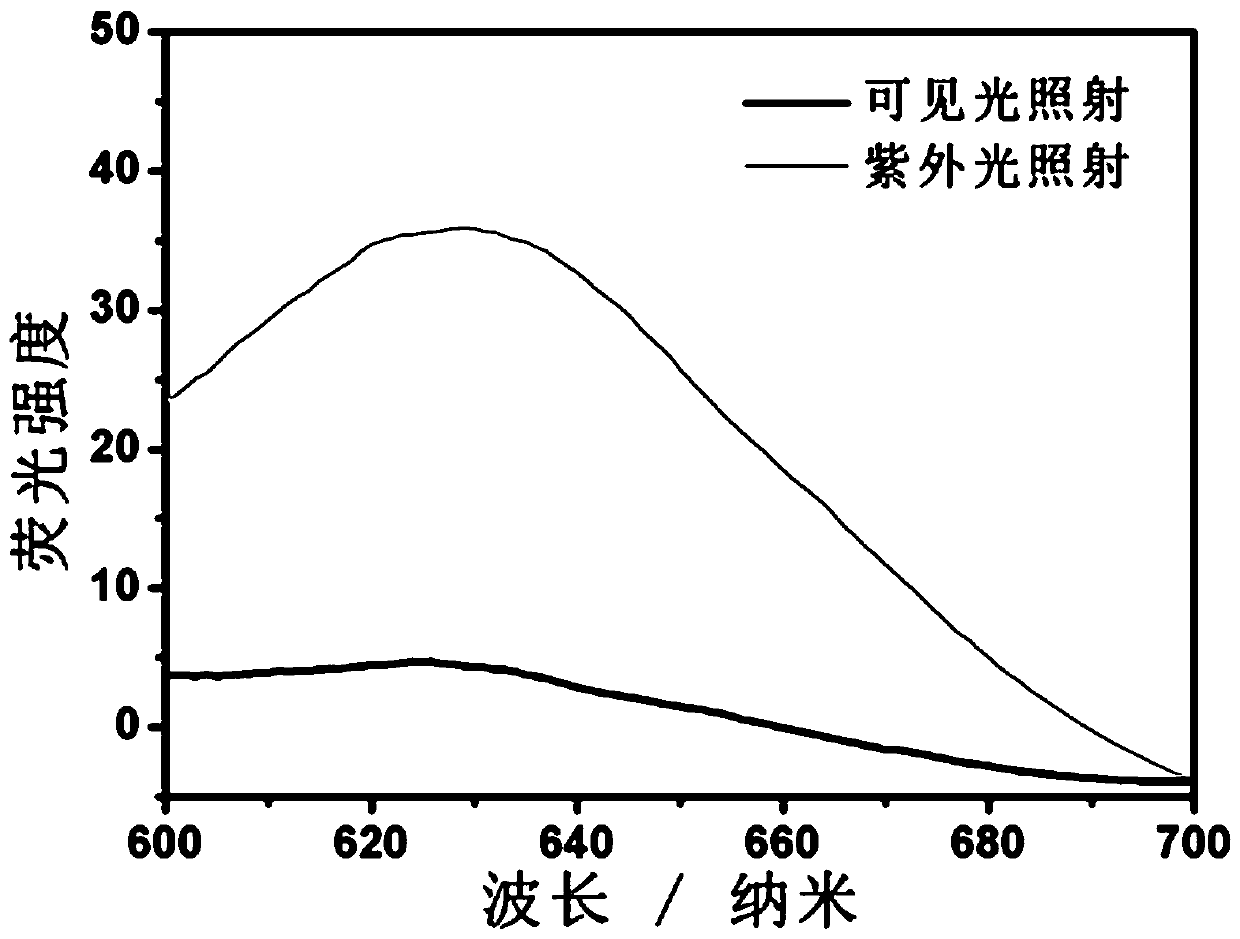
A chain transfer agent, photochromic technology, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of easy leakage of spiropyran, complicated synthesis, difficulty in introducing spiropyran, etc. It is beneficial to large-scale industrial production, simple preparation, and good photochromic properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
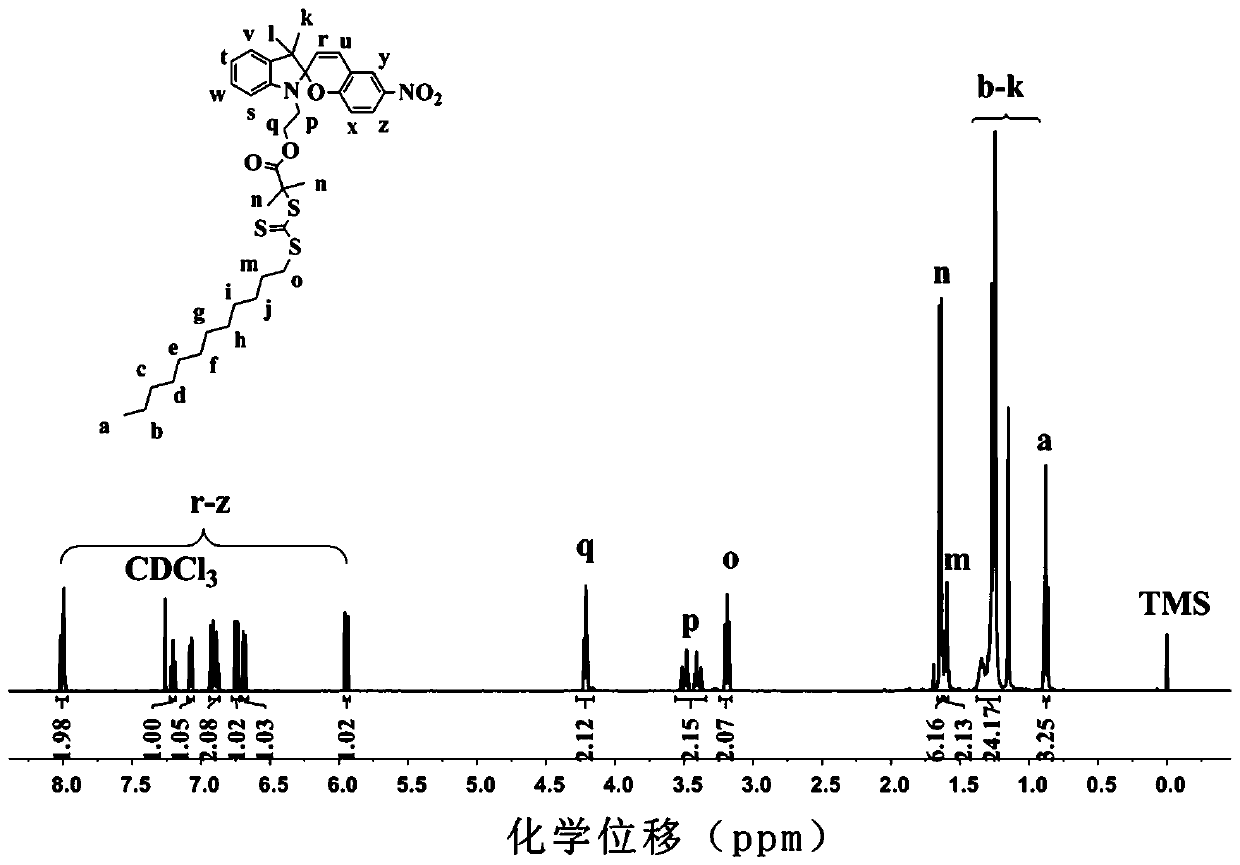
[0027] Embodiment 1: a kind of preparation method of photochromic chain transfer agent, concrete steps are as follows:
[0028] N-hydroxyethyl-3,3-dimethyl-6'-nitroindoline spiropyran 100mg (0.28mmol), 2-(dodecyltrithiocarbonate)-2-iso Add 112mg (0.31mmol) of butyric acid and 7mg (0.06mmol) of 4-dimethylaminopyridine into a 25ml two-neck round-bottomed flask, then add 5ml of dry dichloromethane and stir to dissolve, then stir for 0.5 Hour.
[0029] (2) Add 75 mg (0.33 mmol) of dicyclohexylcarbodiimide to 5 ml of dry dichloromethane to dissolve, then transfer to a constant pressure dropping funnel.
[0030] (3) Slowly add the solution obtained in step (2) to the solution obtained in step (1) dropwise, and react in the dark at room temperature for 24 hours after the addition is completed.
[0031] (4) Suction filter the mixed liquid after reaction to remove the generated white solid. After the resulting solution was concentrated, it was further purified by column chromatograp...
Embodiment 2
[0032] Embodiment 2: a kind of preparation method of photochromic chain transfer agent, concrete steps are as follows:
[0033] N-hydroxyethyl-3,3-dimethyl-5-chloro-6'-nitroindoline spiropyran 110mg (0.28mmol), 2-(dodecyltrithiocarbonate group) - Add 120mg (0.33mmol) of 2-isobutyric acid and 13mg (0.11mmol) of 4-dimethylaminopyridine into a 25ml two-necked round-bottomed flask, then add 5ml of dry dichloromethane and stir to dissolve. Stirred under the same conditions for 0.5 hours.
[0034] (2) Add 70mg (0.31mmol) of dicyclohexylcarbodiimide to 5ml of dry dichloromethane to dissolve, then transfer to a constant pressure dropping funnel.
[0035] (3) Slowly add the solution obtained in step (2) to the solution obtained in step (1) dropwise, and react in the dark at room temperature for 24 hours after the addition is completed.
[0036] (4) Suction filter the mixed liquid after reaction to remove the generated white solid. After the resulting solution was concentrated, it wa...
Embodiment 3
[0037] Embodiment 3: a kind of preparation method of photochromic chain transfer agent, concrete steps are as follows:
[0038]N-hydroxyethyl-3,3-dimethyl-6'-nitroindoline spiropyran 100mg (0.28mmol), 2-(tetradecyltrithiocarbonate)-2-iso Add 137mg (0.36mmol) of butyric acid and 9mg (0.08mmol) of 4-dimethylaminopyridine into a 25ml two-necked round-bottomed flask, then add 5ml of dry dichloromethane and stir to dissolve, then stir in the dark and in an ice bath 0.5 hours.
[0039] (2) Add 81 mg (0.36 mmol) of dicyclohexylcarbodiimide to 5 ml of dry dichloromethane to dissolve, then transfer to a constant pressure dropping funnel.
[0040] (3) Slowly add the solution obtained in step (2) to the solution obtained in step (1) dropwise, and react in the dark at room temperature for 24 hours after the addition is completed.
[0041] (4) Suction filter the mixed liquid after reaction to remove the generated white solid. After the resulting solution was concentrated, it was further...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com