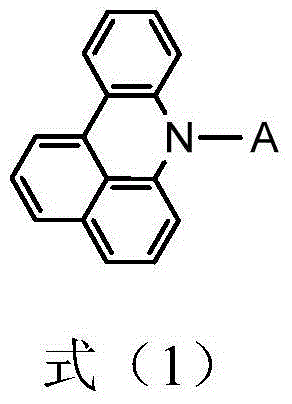
Organic electroluminescent material and preparation method thereof
An electroluminescent material and luminescence technology, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems that the luminous efficiency of luminescent materials cannot meet the requirements of OLEDs, etc., and achieve improved luminous efficiency, high purity, and synthesis and purify simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
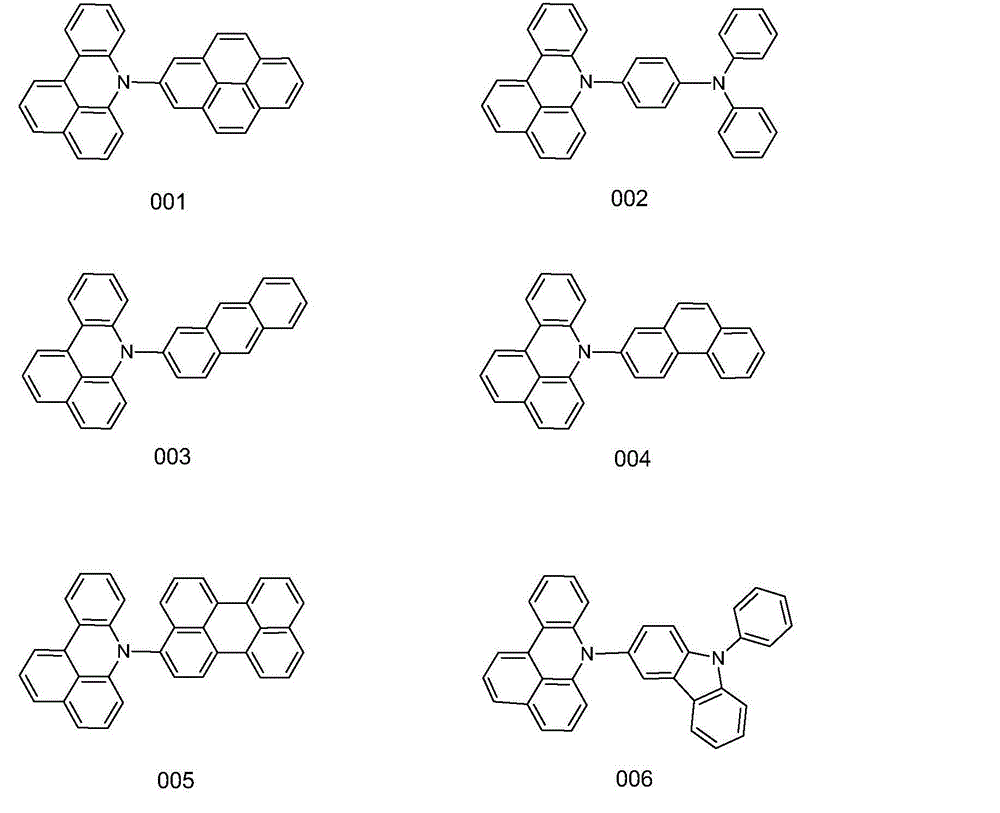
[0027] Embodiment 1: the synthesis of compound 001
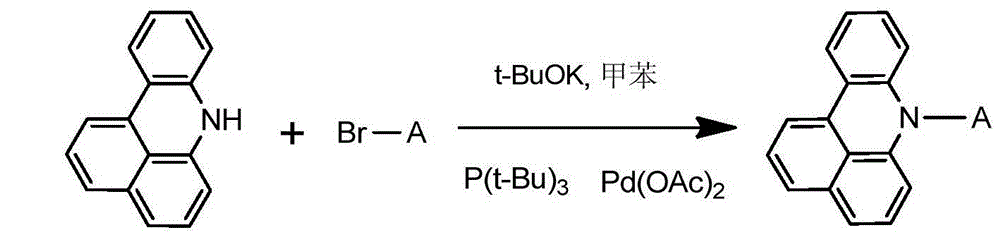
[0028] Concrete synthetic route is as follows:
[0029]
[0030] Weigh 21.72g of 7H-benzoacridine, 42.47g of 2-bromopyrene, 13.46g of potassium tert-butoxide, 0.41g of palladium (II) acetate, and 0.45g of tri-tert-butylphosphine, and dissolve them in 250ml of toluene, under nitrogen protection , reacted at 80°C for 10 hours. The reaction solution was filtered, the obtained crude product was purified by silica gel chromatography, and the obtained solid was recrystallized with toluene and dried to obtain 35.48 g of yellow-white solid compound 001 with a yield of more than 85% and an HPLC purity of more than 98%. Mass Spectrum: Calculated 417.50; Found 417.52. Elemental analysis: calculated value C: 92.06%; H: 4.59%; N: 3.35%; tested value C: 92.07%; H: 4.60%; N: 3.33%.
Embodiment 2
[0031] Embodiment 2: the synthesis of compound 002
[0032] Concrete synthetic route is as follows:
[0033]
[0034] Weigh 21.72g of 7H-benzoacridine, 51.87g of 4-bromotriphenylamine, 14.36g of potassium tert-butoxide, 0.48g of palladium (II) acetate, and 0.54g of tri-tert-butylphosphine, dissolve in 250ml of toluene, and store at 82°C React for 11 hours. The reaction solution was filtered, the obtained crude product was purified by silica gel chromatography, the obtained solid was recrystallized with toluene, and dried to obtain 39.15 g of yellow-white solid compound 002 with a yield of more than 85% and an HPLC purity of more than 98%. Mass Spectrum: Calculated 460.57; Found 460.56. Elemental analysis: calculated value C: 88.67%; H: 5.25%; N: 6.08%; tested value C: 88.69%; H: 5.24%; N: 6.07%.
Embodiment 3
[0035] Embodiment 3: the synthesis of compound 003
[0036] Concrete synthetic route is as follows:
[0037]
[0038] Weigh 21.72g of 7H-benzoacridine, 43.71g of 2-bromoanthracene, 15.26g of potassium tert-butoxide, 0.56g of palladium (II) acetate, and 0.63g of tri-tert-butylphosphine, dissolve in 250ml of toluene, and react at 84°C 12 hours. The reaction solution was filtered, the obtained crude product was purified by silica gel chromatography, the obtained solid was recrystallized with toluene, and dried to obtain 33.84 g of yellow-white solid compound 003 with a yield of more than 86% and an HPLC purity of more than 98%. Mass spectrum: calculated value 397.51; found value 397.52. Elemental analysis: calculated value C: 90.64%; H: 5.83%; N: 3.52%; tested value C: 90.62%; H: 5.84%; N: 3.53%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com