A method for detecting bacterial endotoxin content in recombinant porcine interferon α1 freeze-dried powder injection
A technology of bacterial endotoxin and freeze-dried powder injection, which is applied in the field of microorganisms to achieve the effects of low detection cost, easy operation, and accurate and reliable detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
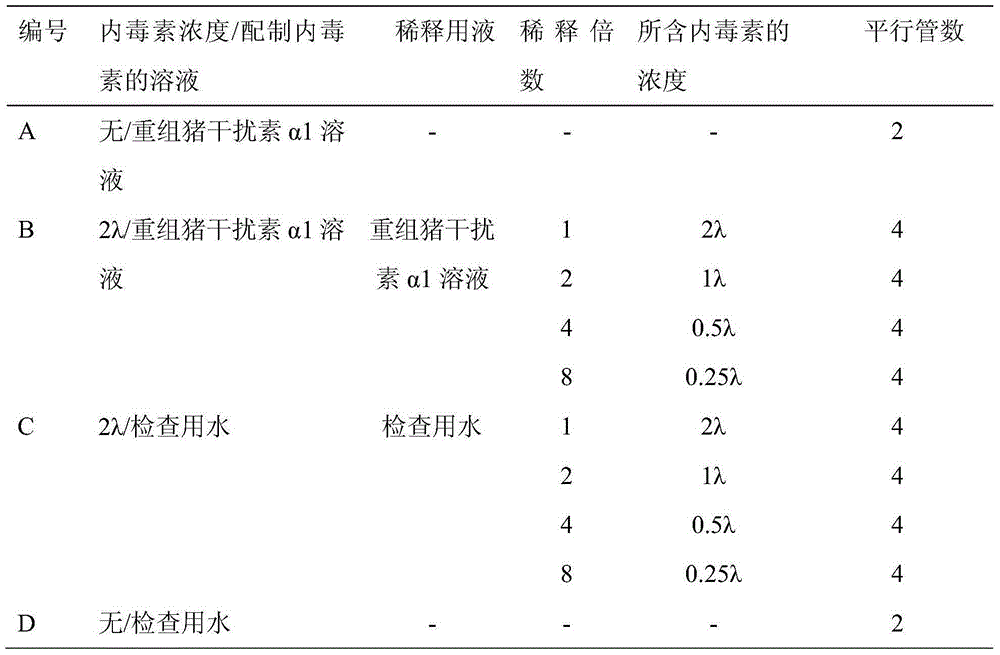
[0013] 1. Reagents
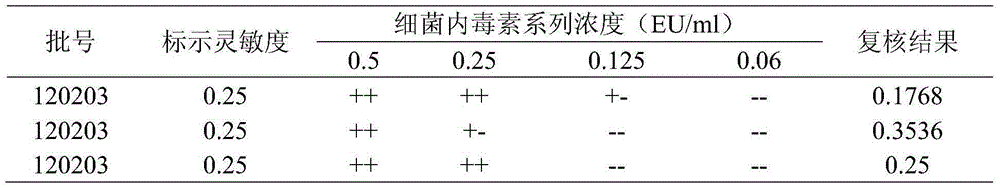
[0014] Test product: Recombinant porcine interferon α1 freeze-dried powder injection (batch number 20121116, potency 1.0×10 6 IU, endotoxin content is 25EU)
[0015] Bacterial endotoxin test water: sterile water for injection with endotoxin content less than 0.015EU / ml and no interference to endotoxin test
[0016] National Standard Bacterial Endotoxin: Purchased from China National Institutes for Food and Drug Control, 10,000 EU each.
[0017] Limulus reagent: purchased from Xiamen Limulus Reagent Factory, 0.1ml / tube (λ=0.25EU / ml)
[0018] 2. Instruments and equipment
[0019] Vortex mixer, parafilm, test tube (10×75mm), pipette, tweezers (metal), metal box.
[0020] All glassware used in the experiment is generally soaked in lotion, then taken out and rinsed with tap water and purified water in sequence (purified water can be used for soaking).
[0021] The utensils used in the test need to be dry-baked at 250°C for more than 30 minutes before use t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com