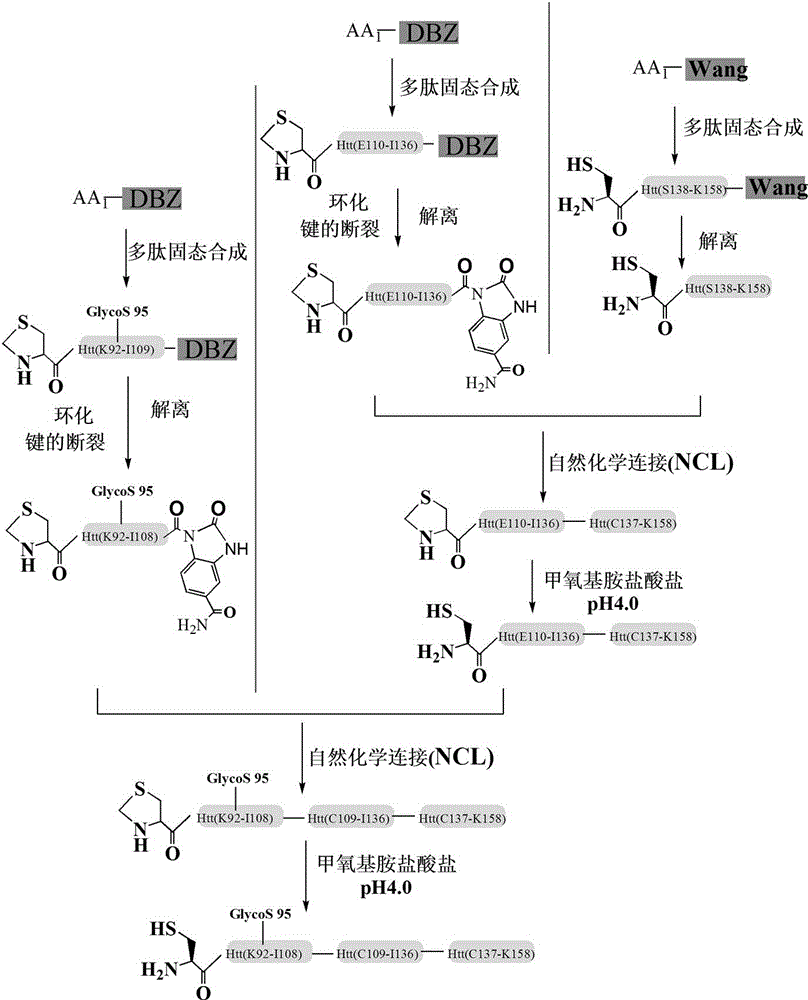
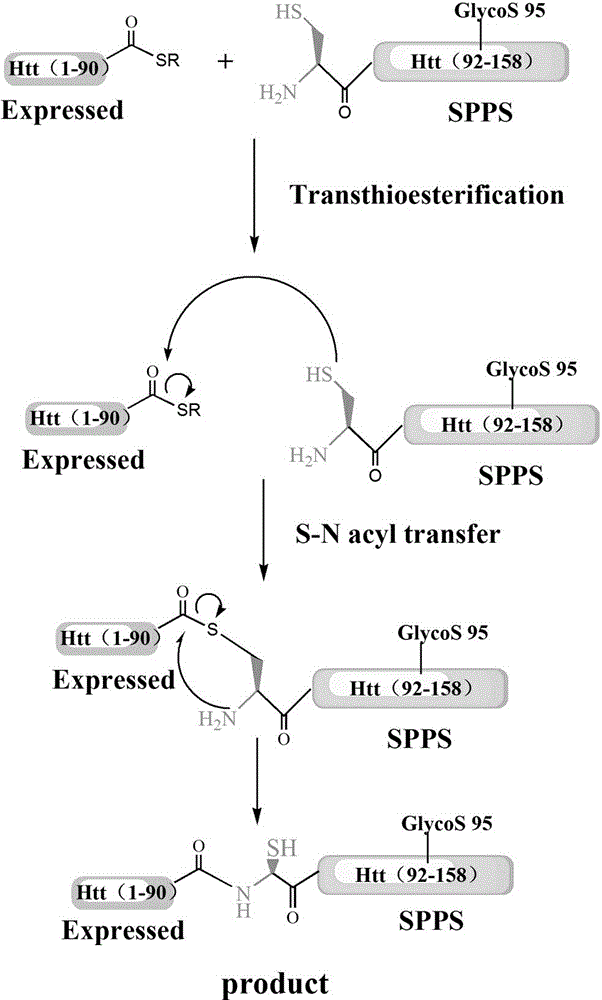
Glycosylation modification method of huntingtin protein
A technology of huntingtin protein and modification method, which is applied in the field of chemical modification of protein, and can solve problems such as poor stability of huntingtin protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
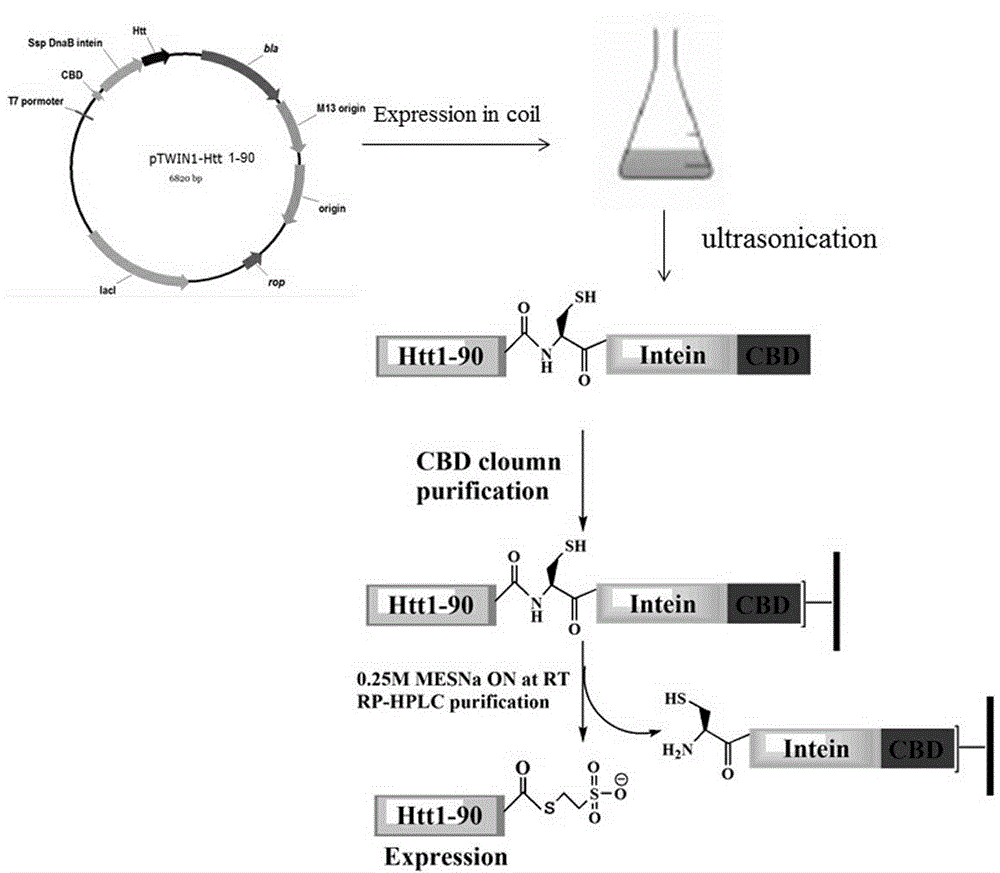
[0027] Example 1: Obtaining recombinant protein Htt1-90
[0028] 1) Test material
[0029] (1) Vector and strain
[0030] Plasmid pcDNA3.1 / myc-His was donated by CHDI; expression vector pTWIN1 was purchased from NEB Company; plasmid pMD18-T and Escherichia coli JM109, DH5α were purchased from Takara Biotechnology Co., Ltd.
[0031] (2) Primers
[0032] P1: 5-CCG GAATTC CTGCCGTGCC-3 (EcoR I)
[0033] P2: 5-AAAA CTGCAG ACAGCCGGGC-3 (Pst I)
[0034] Synthesized by Shanghai Sangon Bioengineering Co., Ltd.
Embodiment approach
[0036] 1. Obtaining the Huntingtin Gene
[0037]Using the plasmid pcDNA3.1 / myc-His as a template, design and synthesize upstream and downstream primers P1 and P2 containing EcoR I and Pst I restriction enzyme sites to amplify the htt gene fragment by PCR, and detect the PCR product by agarose gel electrophoresis Recovered, purified and ligated with vector pMD18-T, transformed into Escherichia coli JM109, and screened for ampicillin resistance to obtain recombinant plasmid pMD18T-htt, which was sent to Shanghai Sangon Bioengineering Co., Ltd. for sequencing. The verified recombinant plasmid was digested with EcoR I and Pst I, and the digested product was purified and stored in a refrigerator at 4°C.
[0038] 2. Construction of recombinant expression plasmid pTWIN1-htt
[0039] The prokaryotic expression vector pTWIN1 was double-digested with restriction endonucleases EcoR I and Pst I, and after purification, the linear pTWIN1 plasmid fragment and the htt gene fragment were lig...
Embodiment 2
[0043] Embodiment 2: Purification of recombinant protein Htt(1-90)
[0044] The CBD component of the Htt1-90-intein-CBD fusion protein can be specifically combined with chitin resin to facilitate the removal of foreign proteins, and then the intein component catalyzes the fusion protein to break between htt1-90 and intein under certain conditions. The specific operation is as follows: the supernatant is loaded on a 2ml chitin gravity column. The column was first equilibrated with solution A (Na-HEPES (pH8.0) 20mmol / L, NaCl 500mmol / L, EDTA 1mmol / L), and the supernatant was loaded and then eluted with solution A. Then the column was immersed in solution B (Na-HEPES (pH8.0) 20mmol / L, NaCl 500mmol / L, EDTA 1mmol / L, DTT 50mmol / L) at 40C for 16h. Solution C (Na-HEPES (pH8.0) 20mmol / L, NaCl 500mmol / L) eluted protein and collected every 1ml. The samples collected in each step were analyzed by SDS-PAGE, and the purity of the identified protein was 90%.
[0045] A solution containing ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com