Optically sensitive pure S-(-)-arotinolol acid salt, preparation method and applications thereof
A technology of acid salt and hydrochloride, which is applied in the field of medicine and can solve the problems of not providing a preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
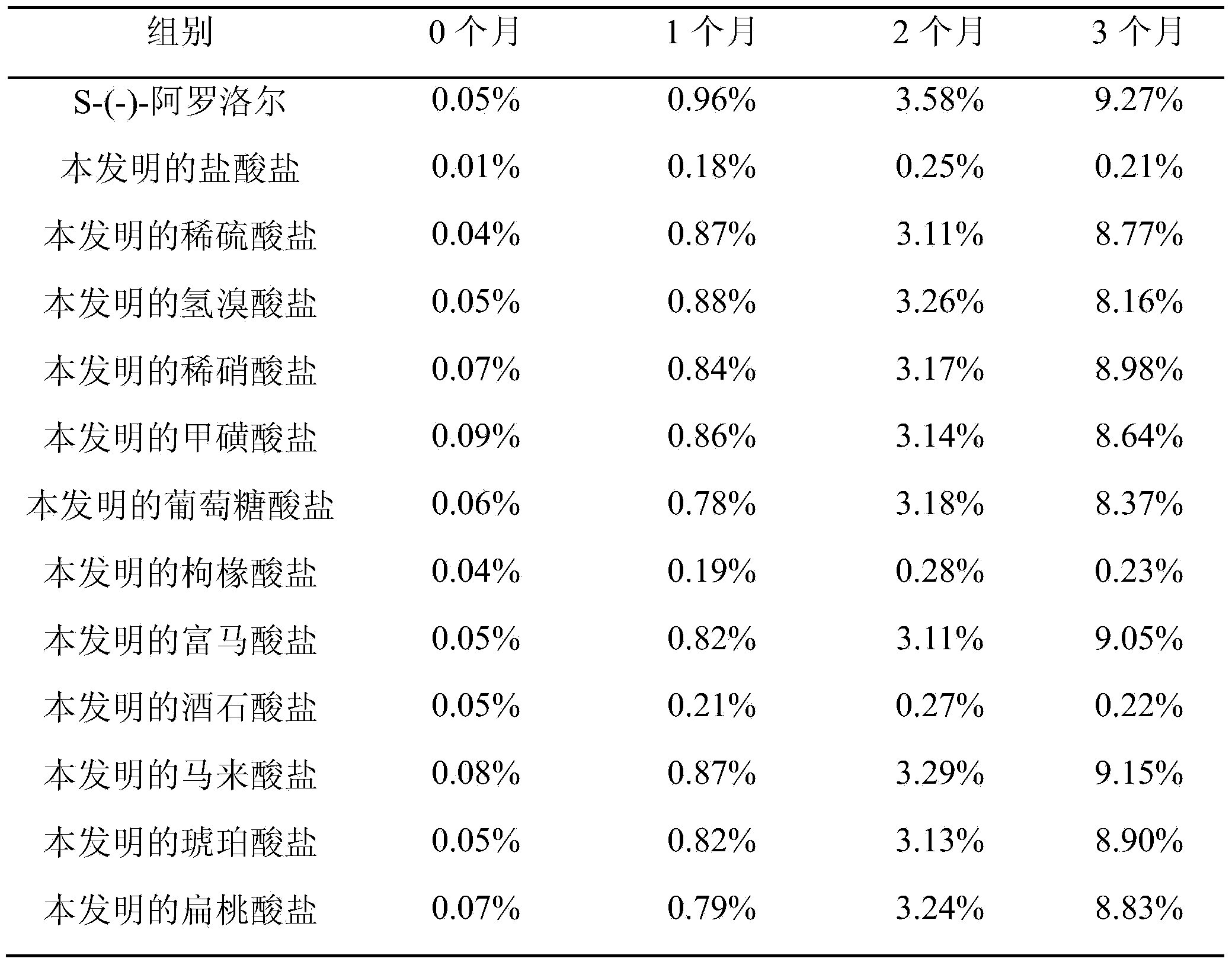
Image
Examples
Embodiment 1
[0045] Example 1 Inorganic acid salt S-(-)-alolol syrup of the present invention
[0046] Dissolve 30 g of sucrose and 1 g of sucralose in 60 ml of warm water, add 1 g of inorganic acid salt S-(-)-alolol after cooling, and add 0.05 g of orange flavoring agent, and dilute the mixture with water to obtain 100 ml of syrup.
Embodiment 2
[0047] Example 2 Organic acid salt S-(-)-alolol syrup of the present invention
[0048] Dissolve 30 g of sucrose and 1 g of sucralose in 60 ml of warm water, add 1 g of organic acid salt S-(-)-alolol after cooling, and add 0.05 g of orange flavoring agent, and dilute the mixture with water to obtain 100 ml of syrup.
Embodiment 3
[0049] Example 3 Inorganic acid salt S-(-)-arololol enteric-coated tablet of the present invention
[0050] Take 100g of inorganic acid salt S-(-)-alolol, mix with 500g of lactose monohydrate, 200g of microcrystalline cellulose, and 50g of hydroxypropyl cellulose, add an appropriate amount of aqueous solution to granulate, and use a swinging granulator to pass 18 mesh screen and dried in an oven. After drying, use a 20-mesh sieve to sieve the granules and mix with 8 g of magnesium stearate. Compress the dry mixture into tablet cores with a punch of 5 mm diameter in a tablet machine, and in a coating device, 200 g of cellulose acetate phthalate and 15 g of cetyl alcohol in 2000 g of isopropanol and 2000 g of dichloromethane Spray the mixed solution on the tablet core to make enteric-coated tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com