Chemiluminescent enzyme-linked immunosorbent assay kit for detecting lipoprotein-associated phospholipase a2 and preparation method thereof
A chemiluminescence enzyme and immunoreagent technology, which is applied in the detection field of chemiluminescence enzyme-linked immunosorbent technology, can solve the problem of not seeing human Lp-PLA2 kits, etc., and achieve the effect of increasing detection specificity and enhancing difference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1. Preparation of Chemiluminescent ELISA Kit for Detection of Lipoprotein-Associated Phospholipase A2 and Its Detection Method
[0029] 1. Preparation of chemiluminescent ELISA kit for detection of lipoprotein-associated phospholipase A2
[0030] 1. Preparation of sample treatment solution
[0031] Sample treatment solution formula: composed of A solution and B solution, wherein A solution is 7.5g ammonium chloride, 1.2g casein, 1.2g sodium stearate, add purified water to make up to 1L, that is, A solution contains 7.5g / L ammonium chloride, 1.2g / L casein and 1.2g / L sodium stearate; B solution is 0.5M Tris-HCl solution (PH7.6).
[0032] 2. Coating anti-Lp-PLA2 monoclonal antibody as a chemiluminescence reaction plate
[0033] The formula of the following antibody coating solution is:
[0034] 0.36g NaH 2 PO 4 ·H 2 O, 3.10 g Na 2 HPO 4 2H 2 O, dilute to 1L, pH7.6.
[0035] Milky white opaque polystyrene 96-well chemiluminescent microtiter plate (Porvair...
Embodiment 2
[0069] Embodiment 2, the test kit that contains sample treatment solution is compared with the test kit that does not contain sample treatment solution
[0070] Experimental group: samples were processed by the sample treatment solution of the present invention
[0071] Control group: samples were not treated.
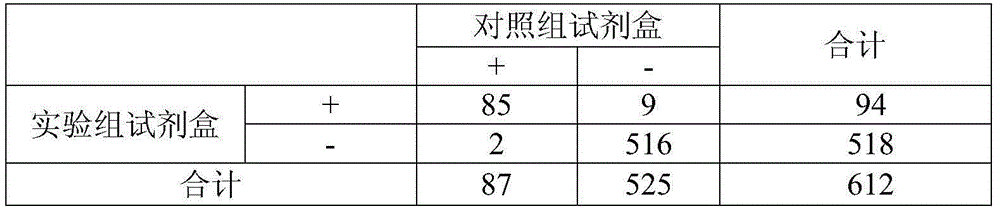
[0072] The comparative experiment was carried out with the above-mentioned experimental group and control group. 100 samples of normal control and 100 samples of cardiovascular and cerebrovascular diseases were tested in parallel by the experimental group and the control group. The detection method was referred to the above-mentioned Example 1. The detection procedures and results were judged in strict accordance with the instructions of each reagent. The test results are shown in Table 1, Table 2 and Table 3:
[0073] Table 1 Test results of the control group kit
[0074] group
Number of cases
+
-
Positive rate (%)
normal control gro...
Embodiment 3
[0080] Embodiment 3, kit sensitivity and accuracy test
[0081] 1. Kit sensitivity experiment
[0082] 1. Carry out 20 tests on the zero standard solution, and the average value of the test results plus 3 times the standard deviation is used as the minimum detection limit of the kit.
[0083] Table 4 Zero standard measurement results statistical table ng / mL
[0084] sample number
1
2
3
4
5
6
7
8
measured value
0.06
0.07
0.08
0.06
0.07
0.05
0.06
0.05
9
10
11
12
13
14
15
16
measured value
0.04
0.05
0.08
0.08
0.06
0.06
0.06
0.07
17
18
19
20
average
standard deviation
Minimum detection limit
measured value
0.06
0.07
0.04
0.06
0.06
0.01
0.1
[0085] It can be seen from Table 4 that the minimum detection limi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com