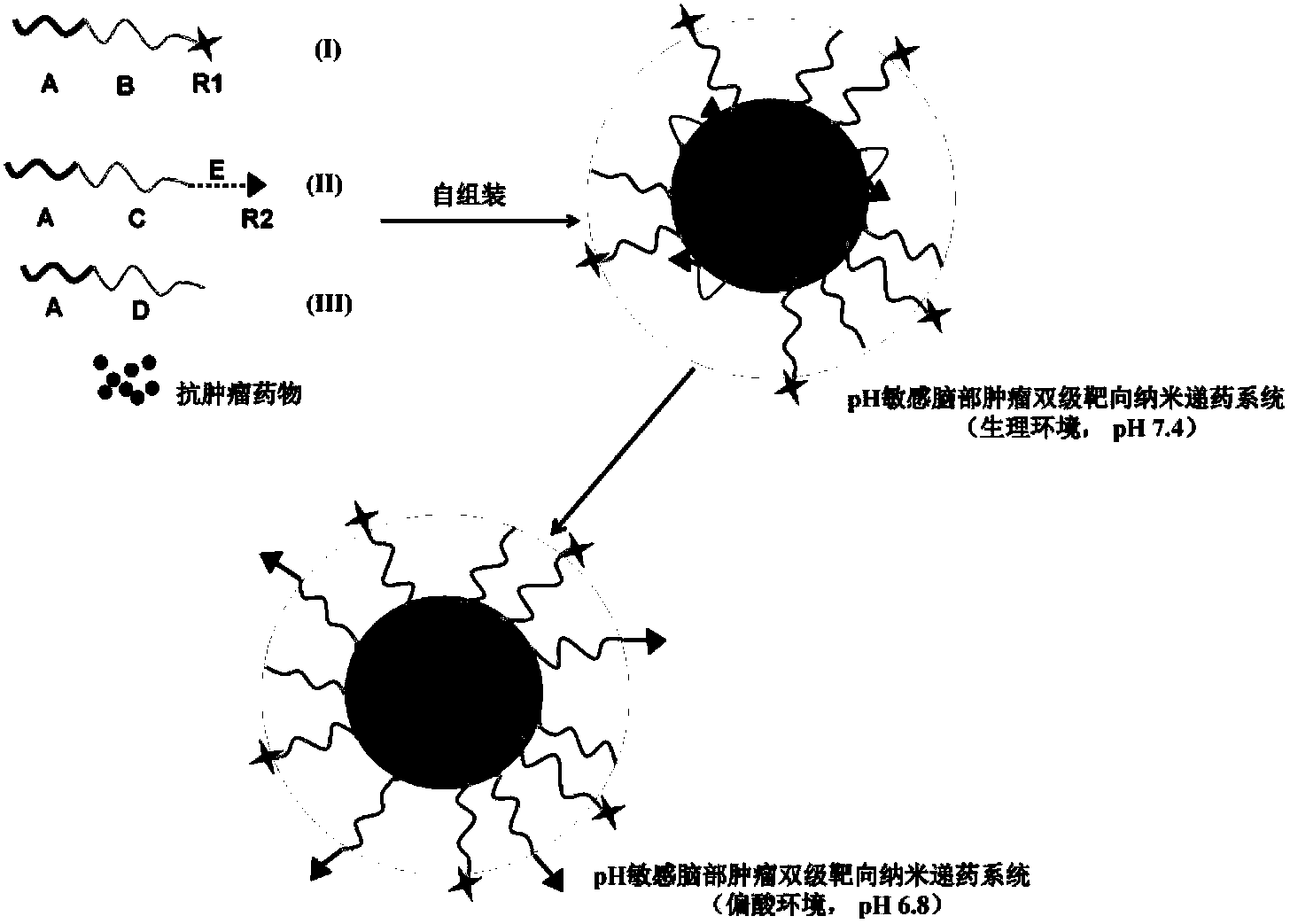
Ph-sensitive brain tumor two-stage targeting NANO drug delivery system, and preparation method and application thereof
A nano-drug delivery system and brain tumor technology, which is applied in anti-tumor drugs, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problem of the preparation method and Application and other issues, to achieve the effect of facilitating subsequent development and industrialization, broad application prospects, and improving the degree of targeted concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of pH-sensitive tumor cell-targeting functional materials (II)
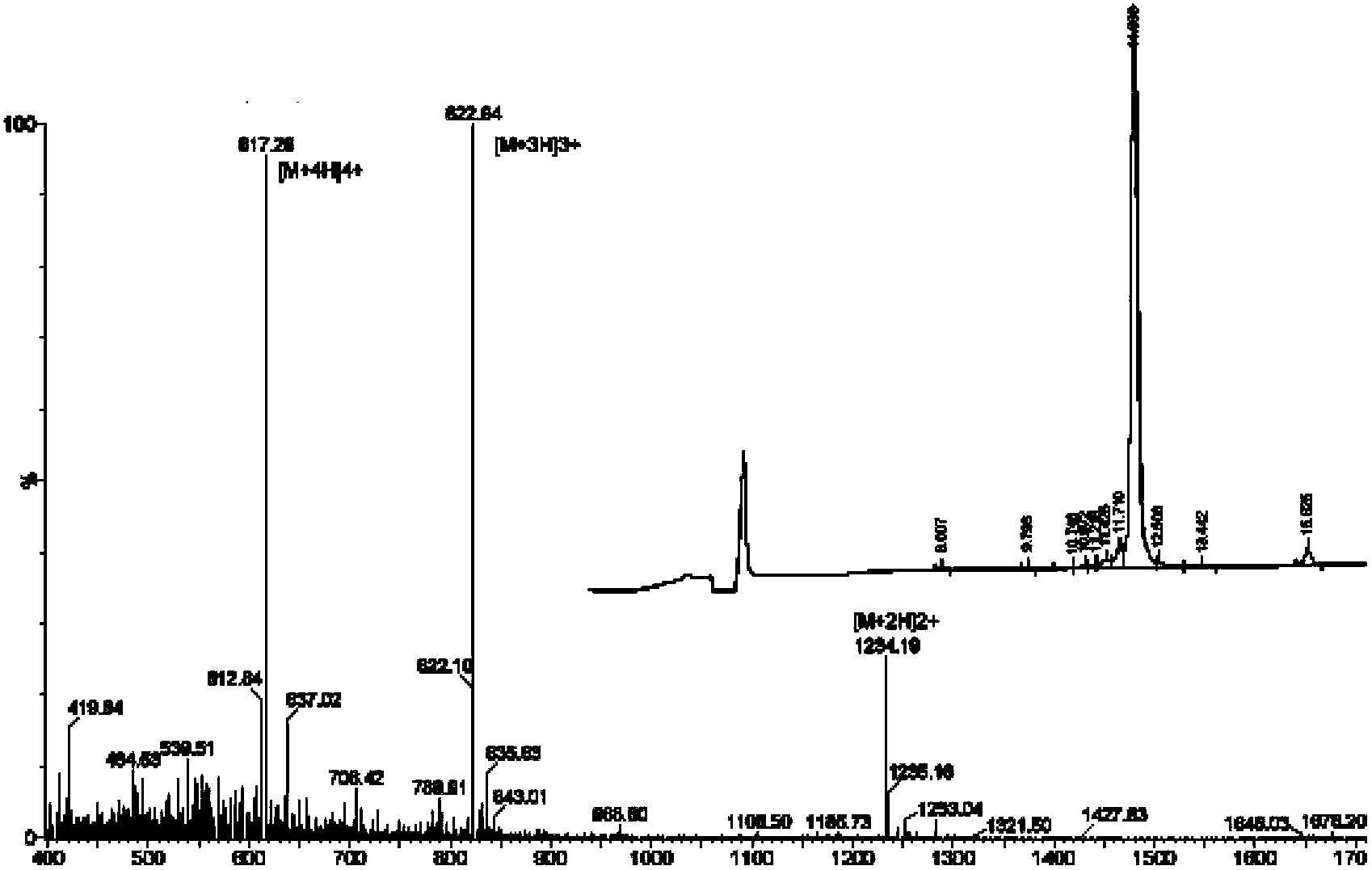
[0056] Using the solid-phase peptide synthesis method, the Rink amino resin was deprotected with trifluoroacetic acid (TFA) for 1 min, and the amino acids were protected with Fmoc to respond sequentially to the RGD peptide of the sequence CRGDKGPDC, and the cysteines at both ends were cyclized to form RDG cyclic peptide c (CRGDKGPDC), further using Fmoc to protect amino acids in the -NH of c(CRGDKGPDC) 2 The end group introduces 10 histidines (poly(histidine) 10 ) and a cysteine; after removing the protective group of the above-mentioned thiolated pH-sensitive polypeptide with piperidine DMF solution, the polypeptide was cleaved from the resin with hydrofluoric acid, and separated and purified with acetonitrile / water (containing 0.1% TFA) ; HPLC and ESI-MS were used to characterize the purity and molecular weight of the pH-sensitive tumor cell targeting polypeptide CC20 (CHHHHHHHHHH-c(CRGDKGPD...
Embodiment 2
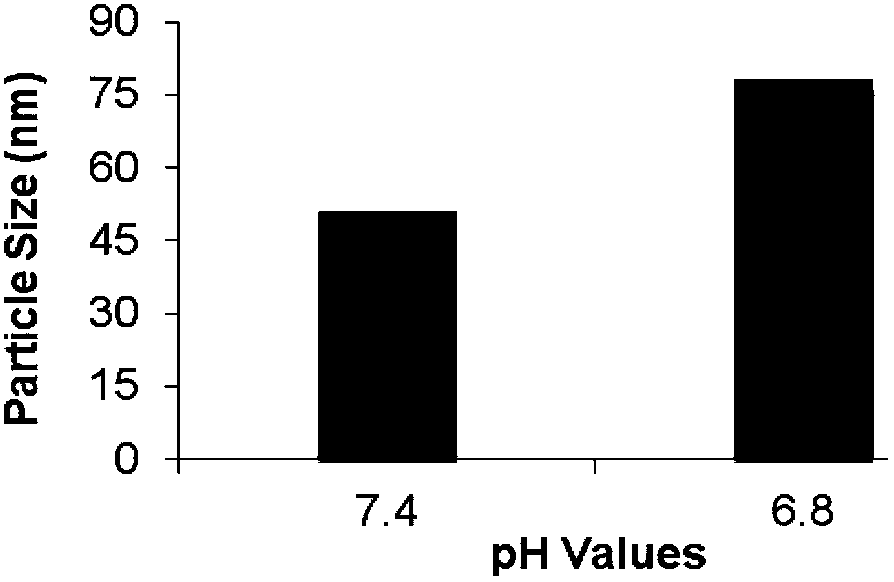
[0060] Preparation of pH-sensitive dual-stage targeting polymeric micelles for brain tumors and evaluation of their pH sensitivity
[0061] Brain targeting molecule R1-angiopep (TFFYGGSRGKRNNFKTEEY) was synthesized by solid-phase peptide synthesis method, and its -NH 2 The end group is connected with cysteine to obtain the sulfhydrylated brain-targeting molecule R1. React it with maleimide-polyethylene glycol-polylactic acid (maleimide-PEG-PLA) in PBS at pH 7.4 overnight, dialyze to remove unreacted small molecules, and freeze-dry to obtain the brain target To the functional material angiopep-PEG-PLA;
[0062] The brain-targeting functional material angiopep-PEG-PLA (1.4 mg), the pH-sensitive tumor cell-targeting functional material CC20-PEG-PLA (1.4 mg) and the amphiphilic polymer material mPEG-PLA (17.2 mg) were dissolved in In 3 mL of acetonitrile, hydrate under reduced pressure to form a film for 2 hours, then use 3 mL of hydration medium for hydration, and filter the ...
Embodiment 3
[0064] Example 3 In vivo targeting evaluation of pH-sensitive brain tumor dual-stage targeting polymer micelles
[0065] Referring to the method described in Example 1, a pH-sensitive tumor cell targeting functional material CC30-PEG-PLA was synthesized, wherein the pH-sensitive molecule poly(histidine) 20 Contains 20 histidines.
[0066] The brain-targeting functional material angiopep-PEG-PLA (1.4mg), the pH-sensitive tumor cell-targeting functional material CC30-PEG-PLA (1.4mg) and the amphiphilic polymer material mPEG-PLA (17.2mg) were dissolved in Add 15 μg of near-infrared probe DiR to 3 mL of acetonitrile, hydrate under reduced pressure to form a film for 2 hours, use 3 mL of hydration medium for hydration, and filter the obtained polymer micelles with a 0.45 μm microporous membrane to obtain pH-sensitive brain tumors Two-stage targeting polymer micelles angio-PEG-PLA / CC30-PEG-PLA; ordinary mPEG-PLA micelles without targeting functional materials and brain targeting an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com