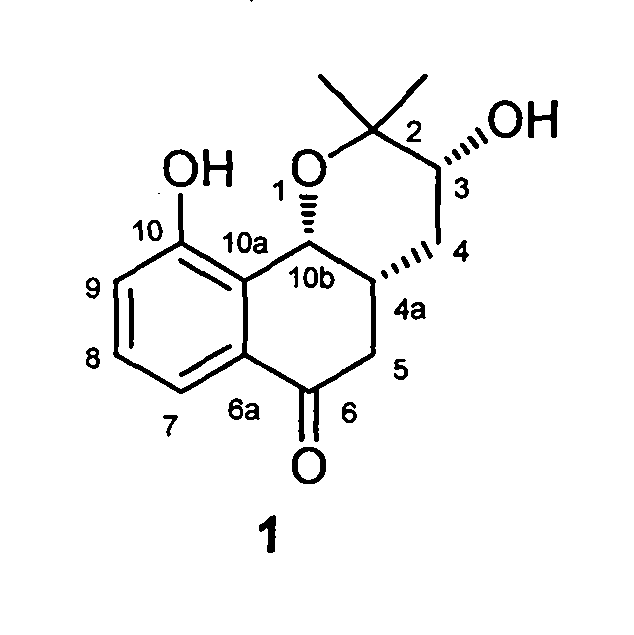
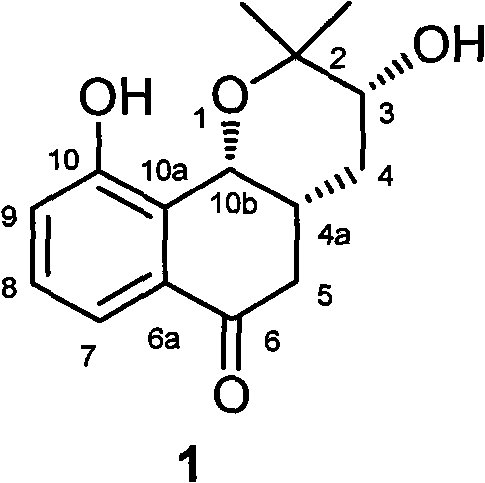
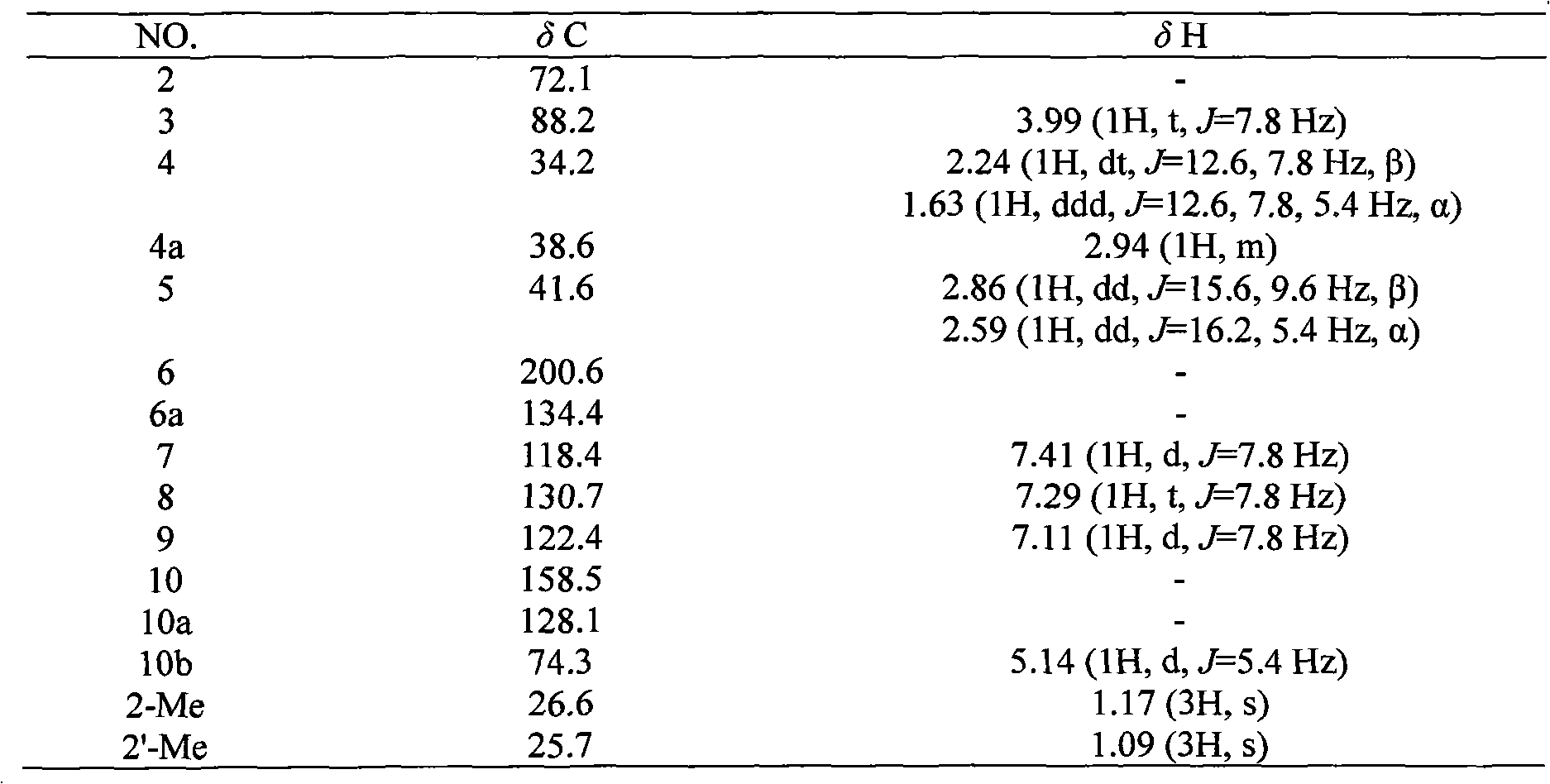
Novel isopentenyl naphthoquinone obtained in active fraction for promoting blood circulation to remove blood stasis in clinopodium chinense (Benth.) O. Kuntze. and preparation method thereof
A technology of isopentenyl naphthoquinone, promoting blood circulation and removing blood stasis, which is applied to medical preparations containing active ingredients, organic chemistry, pharmaceutical formulas, etc., and can solve problems such as no patents or literature reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0018] Example: Compound Preparation.
[0019] Take the dry aboveground part of the spinach, cleanly select, cut into sections, and extract with water. The extract obtained after concentrating and drying the extract is subjected to macroporous resin chromatography with ethanol-water (0%-95%) as the eluent to obtain 50-95% eluate, which is enriched by alkali extraction and acid precipitation. Collected and refined to obtain the total flavonoids of Campanula chinensis. The total flavonoids of Campanula chinensis were roughly separated by silica gel column chromatography, and gradient elution was carried out with a mixed solvent of chloroform-methanol in different proportions, wherein chloroform:methanol (97:3) eluted fractions 12-48 were subjected to reverse-phase ODS column chromatography Separation, eluting with methanol-water gradient, where methanol: water (40:60) eluted part was separated by Sephadex LH-20 gel column, eluted with methanol, and fraction 3 was collected and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com