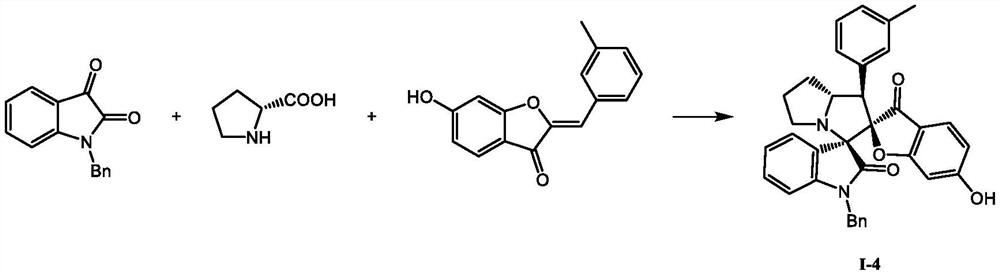
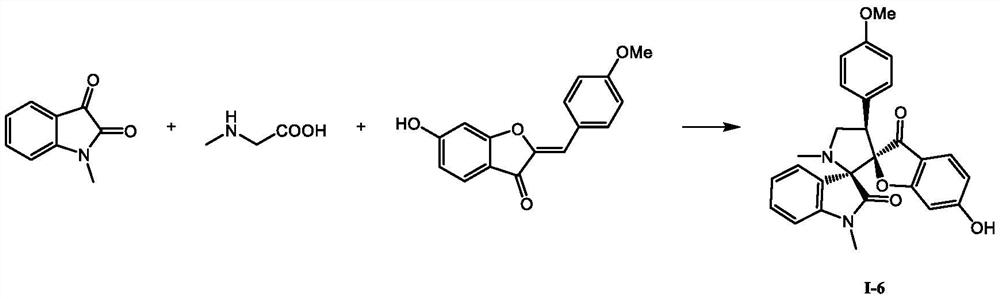
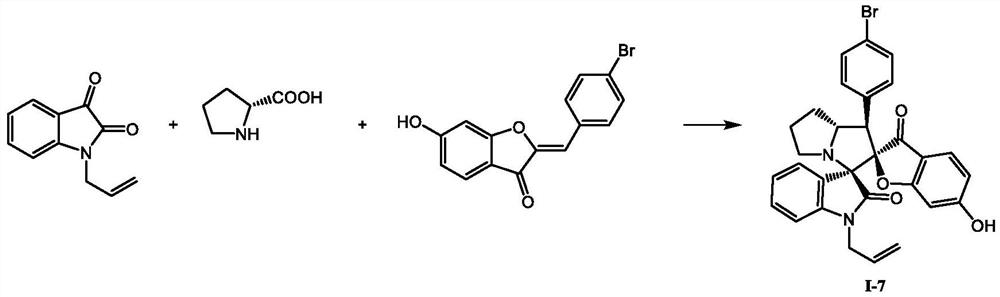
A kind of spiro indolinone compound and its preparation method and application
A compound, orange ketone technology, applied in the field of spirocyclic indolinone compounds and their preparation, can solve problems such as blanks and lack of research results, and achieve the effects of convenient post-processing, expanding application scope and unique chemical structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Weigh 0.0886g N-methylisatin (0.55mmol), 0.0588g sarcosine (0.66mmol) and 0.1191g 6-hydroxyaurone (0.5 mmol) and dissolve them in 4mL of methanol, stir at room temperature for 3h, detect by TLC, confirm The reaction has been completed, the methanol solvent is removed under reduced pressure, and a white solid is obtained by methanol recrystallization, which has the formula The compound of the structure, the yield is 65%. The compound with the structure (I-1) was determined, and its m.p. was 242.7-243.4°C; 1 H NMR (400 MHz, DMSO-d 6 )δ: 10.86(s, 1H), 7.37~7.35(m, 2H), 7.28(dd, J=7.6, 1.2Hz, 1H), 7.26~7.18(m, 5H), 6.98(td, J=7.5, 1.0Hz, 1H), 6.84(d, J=7.7Hz, 1H), 6.32(dd, J=8.5, 1.9Hz, 1H), 5.99 (d, J=1.8Hz, 1H), 4.15~4.03(m, 2H), 3.45(t, J=7.5Hz, 1H), 3.06(s, 3H), 2.08(s, 3H); 13 C NMR (100MHz, DMSO-d 6 )δ: 196.7, 173.3, 172.6, 167.0, 144.7, 135.1, 130.0 (2C), 129.6, 127.8 (2C), 127.0, 126.5, 125.5, 123.7, 122.0, 112.1, 111.4, 108.1, 97.2, 946.5, 7 , 51.4, 34.2, ...
Embodiment 2
[0018] Accurately weigh 0.1051g N-ethylisatin (0.60mmol), 0.0633g D-proline (0.55mmol) and 0.1191g 6-hydroxyaurone (0.5mmol) and dissolve in 4mL toluene, stir, and reflux for 48h, TLC detection confirms that the reaction has been completed, the toluene solvent is removed under reduced pressure, and methanol is used to recrystallize to a white solid, which has the formula The compound of the structure, the yield is 66%.
[0019] Determination of the compound with structure (I-2), its m.p.212.0~212.6°C; 1 H NMR (400MHz, DMSO-d 6 )δ:10.90(s,1H),7.48(dd,J=7.6,1.2Hz,1H),7.37~7.35(m,2H),7.27~7.22(m,3H),7.19~7.14(m,2H) ,7.00(td,J=7.6,1.1Hz,1H),6.90(d,J=7.8Hz,1H),6.32(dd,J=8.5,1.9Hz,1H),6.11(d,J=1.9Hz, 1H), 4.78~4.73(m, 1H), 3.75~3.66(m, 1H), 3.61(d, J=10.0 Hz, 1H), 3.53~3.44(m, 1H), 3.35~3.31(m, 1H) ,2.67(t,J=7.7Hz,1H),2.08~1.96(m,2H), 1.87~1.66(m,2H),0.94(t,J=7.0Hz,3H); 13 C NMR (100MHz, DMSO-d 6 )δ:195.7,173.3,172.6,167.0,142.9,134.5,129.9(2C),129.6,128.1,128.0(2C),127.1,1...
Embodiment 3
[0021] Accurately weigh 0.0886g N-methylisatin (0.55mmol), 0.0633g D-proline (0.55mmol) and 0.1261g 6-hydroxy-2'-methyl aurone (0.5mmol) and dissolve in 4mL methanol , heated up to 50°C for 5 hours, TLC detection confirmed the completion of the reaction, removed the methanol solvent under reduced pressure, and recrystallized using methanol and ethyl acetate to obtain a white solid, which has the formula The compound of the structure, the yield is 65%.
[0022] Determination of the compound with structure (I-3), m.p.315.1~315.4°C; 1 H NMR (400MHz, DMSO-d 6 )δ: 10.92(s, 1H), 7.83(d, J=7.6Hz, 1H), 7.50(d, J=7.4Hz, 1H), 7.27(td, J=7.7, 0.8Hz, 1H), 7.22~ 7.18(m, 2H), 7.06~6.99(m, 3H), 6.88(d, J=7.8Hz, 1H), 6.32(dd, J=8.5, 1.9 Hz, 1H), 6.05(d, J=1.8Hz ,1H),4.83~4.78(m,1H),3.99(d,J=9.9Hz,1H),3.54~3.48(m,1H), 3.06(s,3H),2.70(t,J=7.8Hz, 1H), 2.11(s, 3H), 2.07~1.95(m, 2H), 1.82~1.61(m, 2H); 13 C NMR (100MHz, DMSO-d 6)δ: 196.9, 173.8, 172.6, 167.1, 144.3, 136.4, 132.7, 131.0, 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com