Application of macromolecular protein in antibacterial and antiviral disinfectant
A macromolecular and disinfectant technology, applied in the direction of antiviral, disinfectant, antifungal, etc., can solve the problems of lack of understanding, corruption and degradation, variability, etc., to achieve strong stability and activity, long action time, Active lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] An antibacterial and antiviral disinfectant of macromolecular protein, the disinfectant contains one or more recombinant human RNase enzyme family proteins.
[0033] The record entries of human RNases in the Uniprot database are shown in the table below:
[0034]
[0035]
[0036] These proteins act as enzymes and they catalyze the following reactions:
[0037]An(RNA)containing cytidine+H(2)O=an(RNA)-3'-cytidine-3'-phosphate+a5'-hydroxy-ribonucleotide-3'-(RNA)An(RNA)containing uridine+H( 2) O=an(RNA)-3'-uridine-3'-phosphate+a 5'-hydroxy-ribonucleotide-3'-(RNA)
[0038] These proteins are mainly naturally distributed outside the cells of various organs and tissues throughout the body, and are also secreted to the body surface, oral cavity, intestinal tract, and urethra through tears, saliva, mucus, and sweat. As natural immune molecules, they resist pathogenic microorganisms in the human body.
[0039] The RNases in this example are recombinant human-derived RNas...
Embodiment 2
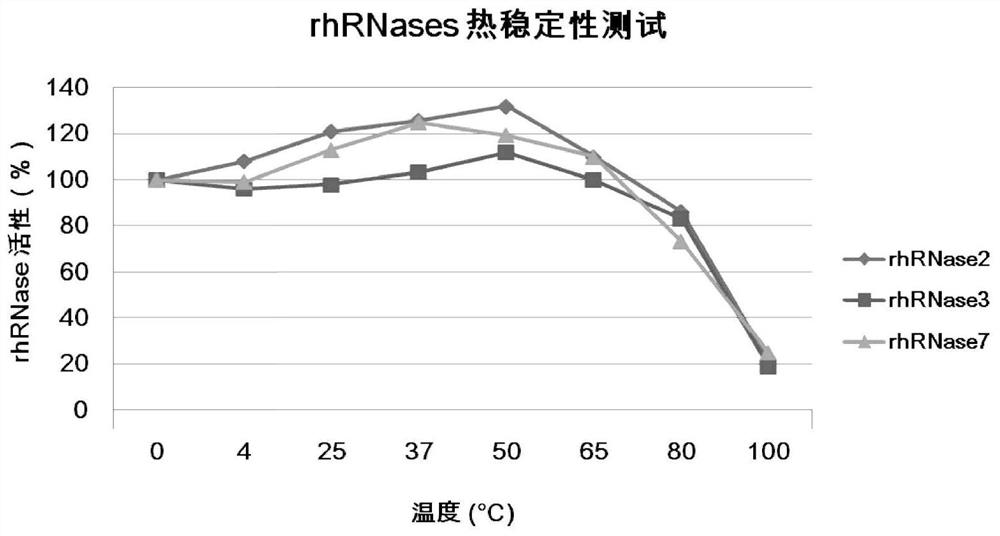
[0046] The stability of embodiment 2 disinfectants
[0047] (1) Thermal stability of recombinant RNases
[0048] Under various temperature conditions, the activity changes of three RNases were tested. The RNase activity detection process is as follows: 40 mg of yeast tRNA is added as a substrate to 0.8 mL of reaction solution (containing 40 mM sodium phosphate, pH 7.0 and 10 mL of rhRNase2, or rhRNase3, or rhRNase7, or buffer control). Reactions were terminated by adding 40 nM lanthanum nitrate in ice-cold 3% perchloric acid at given time points. Afterwards, centrifugation, acid-soluble ribonucleotides remaining in the supernatant were quantified spectrophotometrically at 260 nM. All time points were tested in triplicate. Calculations included the following approximations: tRNA had an average molecular weight (Mr) of Mr = 28,100 (75-90 ribonucleotides / tRNA molecule 1 Mr 341 / ribonucleotides) with an absorbance at 260 nm of 1.0, corresponding to 40 mg RNA. Taking the RNase a...
Embodiment 3
[0062] The bacteriostasis of embodiment 3 disinfectant
[0063] Common clinical pathogenic bacteria and fungi were incubated in 100μl 10mM sodium phosphate buffer (pH 7.4) containing 1% tryptone soybean broth and RNase7 at different concentrations above at 37°C for 3 hours, then transferred to culture Plate overnight, count the colonies (CFU) the next day, and then convert to CFU / mL.
[0064] Test results such as Figure 6 Shown in order are Enterobacter (Escherichia coli), Staphylococcus aureus (Staphylococcus aureus), Pseudomonas aeruginosa (Pseudomonas aeruginosa), Prupionibacterium acnes, Enterococcus faecium (Enterococcus Faecium), Candida albicans (candidaalbicans) statistical graph; As can be seen from the figure, RNase7 has an inactivation effect on all these pathogenic bacteria, and the sensitivity of various pathogenic bacteria to RNase is different, with the highest sensitivity of Enterococcus, Candida albicans (fungi ) has the lowest sensitivity. It is worth not...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com