Spiro indolinone compound, and preparation method and application thereof
The technology of a compound, aurone, is applied in the field of spiro-indolinone compounds and their preparation, which can solve the problems of lack of research results and blanks, and achieve the goals of expanding the application range, convenient post-processing, and good tobacco mosaic virus activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
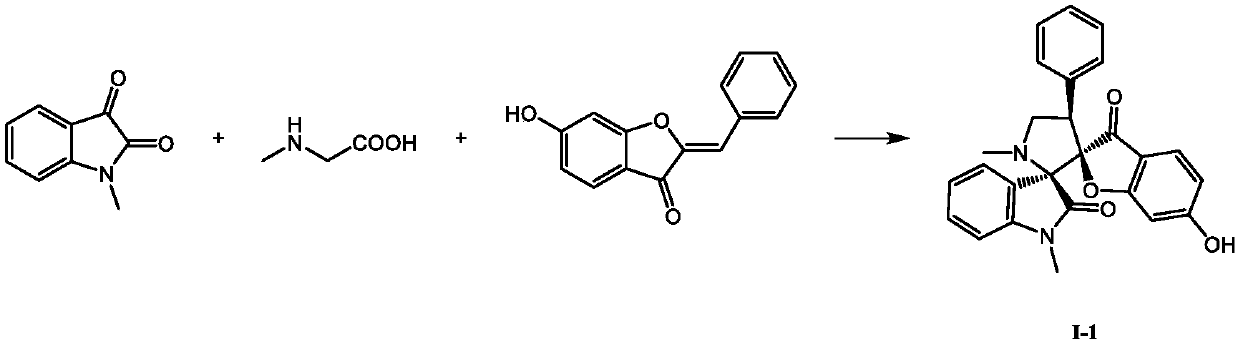
[0016]
[0017] Weigh 0.0886g N-methylisatin (0.55mmol), 0.0588g sarcosine (0.66mmol) and 0.1191g 6-hydroxyaurone (0.5 mmol) and dissolve them in 4mL of methanol, stir at room temperature for 3h, detect by TLC, confirm The reaction has been completed, the methanol solvent is removed under reduced pressure, and a white solid is obtained by methanol recrystallization, which has the formula The compound of the structure, the yield is 65%. The compound with the structure (I-1) was determined, and its m.p. was 242.7-243.4°C; 1 H NMR (400MHz, DMSO-d 6 )δ:10.86(s,1H),7.37~7.35(m,2H),7.28(dd,J=7.6,1.2Hz,1H),7.26~7.18(m,5H),6.98(td,J=7.5, 1.0Hz, 1H), 6.84(d, J=7.7Hz, 1H), 6.32(dd, J=8.5, 1.9Hz, 1H), 5.99 (d, J=1.8Hz, 1H), 4.15~4.03(m, 2H), 3.45(t, J=7.5Hz, 1H), 3.06(s, 3H), 2.08(s, 3H); 13 C NMR (100MHz, DMSO-d 6 )δ: 196.7, 173.3, 172.6, 167.0, 144.7, 135.1, 130.0 (2C), 129.6, 127.8 (2C), 127.0, 126.5, 125.5, 123.7, 122.0, 112.1, 111.4, 108.1, 97.2, 946.5, 7 , 51.4, 34.2, 25....
Embodiment 2
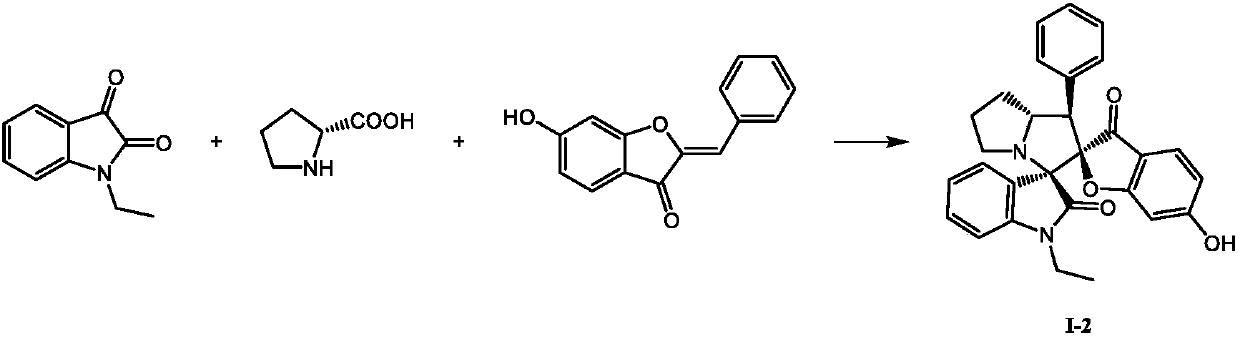
[0019]
[0020] Accurately weigh 0.1051g N-ethyl isatin (0.60mmol), 0.0633g D-proline (0.55mmol) and 0.1191g 6-hydroxyaurone (0.5mmol) and dissolve in 4mL toluene, stir, reflux for 48h, TLC Detect to confirm that the reaction has been completed, remove the toluene solvent under reduced pressure, and use methanol to recrystallize to a white solid, which has the formula The compound of the structure, the yield is 66%.
[0021] Determination of the compound with structure (I-2), its m.p.212.0~212.6°C; 1 H NMR (400MHz, DMSO-d 6 )δ:10.90(s,1H),7.48(dd,J=7.6,1.2Hz,1H),7.37~7.35(m,2H),7.27~7.22(m,3H),7.19~7.14(m,2H) ,7.00(td,J=7.6,1.1Hz,1H),6.90(d,J=7.8Hz,1H),6.32(dd,J=8.5,1.9Hz,1H),6.11(d,J=1.9Hz, 1H), 4.78~4.73(m, 1H), 3.75~3.66(m, 1H), 3.61(d, J=10.0 Hz, 1H), 3.53~3.44(m, 1H), 3.35~3.31(m, 1H) ,2.67(t,J=7.7Hz,1H),2.08~1.96(m,2H), 1.87~1.66(m,2H),0.94(t,J=7.0Hz,3H); 13 C NMR (100MHz, DMSO-d 6 )δ:195.7,173.3,172.6,167.0,142.9,134.5,129.9(2C),129.6,128.1,128.0(2C),127.1,125...
Embodiment 3
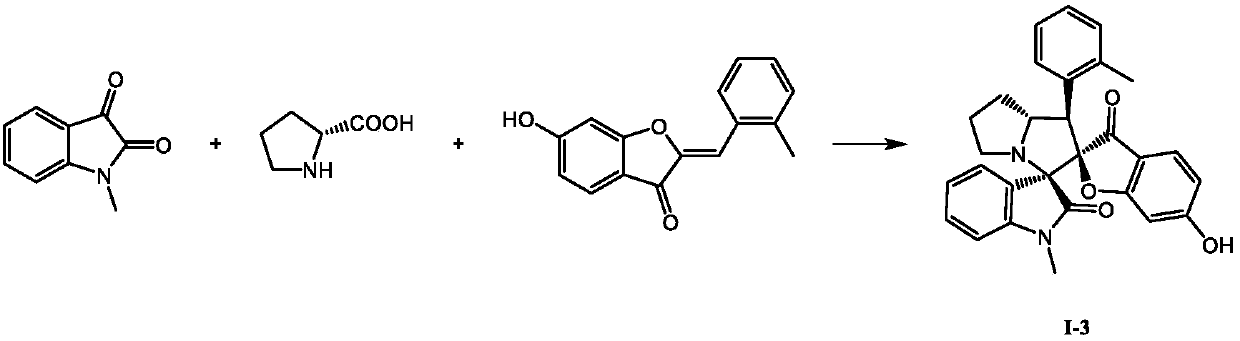
[0023]
[0024] Accurately weigh 0.0886g N-methyl isatin (0.55mmol), 0.0633g D-proline (0.55mmol) and 0.1261g 6-hydroxyl-2'-methyl orangerone (0.5mmol) and dissolve in 4mL methanol, Raise the temperature to 50°C for 5 hours, TLC detection, confirm the completion of the reaction, remove the methanol solvent under reduced pressure, use methanol and ethyl acetate to recrystallize to obtain a white solid, which has the formula The compound of the structure, the yield is 65%.
[0025] Determination of the compound with structure (I-3), m.p.315.1~315.4°C; 1 H NMR (400MHz, DMSO-d 6 )δ: 10.92(s, 1H), 7.83(d, J=7.6Hz, 1H), 7.50(d, J=7.4Hz, 1H), 7.27(td, J=7.7, 0.8Hz, 1H), 7.22~ 7.18(m, 2H), 7.06~6.99(m, 3H), 6.88(d, J=7.8Hz, 1H), 6.32(dd, J=8.5, 1.9 Hz, 1H), 6.05(d, J=1.8Hz ,1H),4.83~4.78(m,1H),3.99(d,J=9.9Hz,1H),3.54~3.48(m,1H), 3.06(s,3H),2.70(t,J=7.8Hz, 1H), 2.11(s, 3H), 2.07~1.95(m, 2H), 1.82~1.61(m, 2H);13 C NMR (100MHz, DMSO-d 6 )δ: 196.9, 173.8, 172.6, 167.1, 144.3, 136...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com