Application of terpenoid coumarins in the treatment of gastric cancer
A technology for gastric cancer and drugs, applied in the fields of active ingredients of heterocyclic compounds, organic chemistry, digestive system, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example
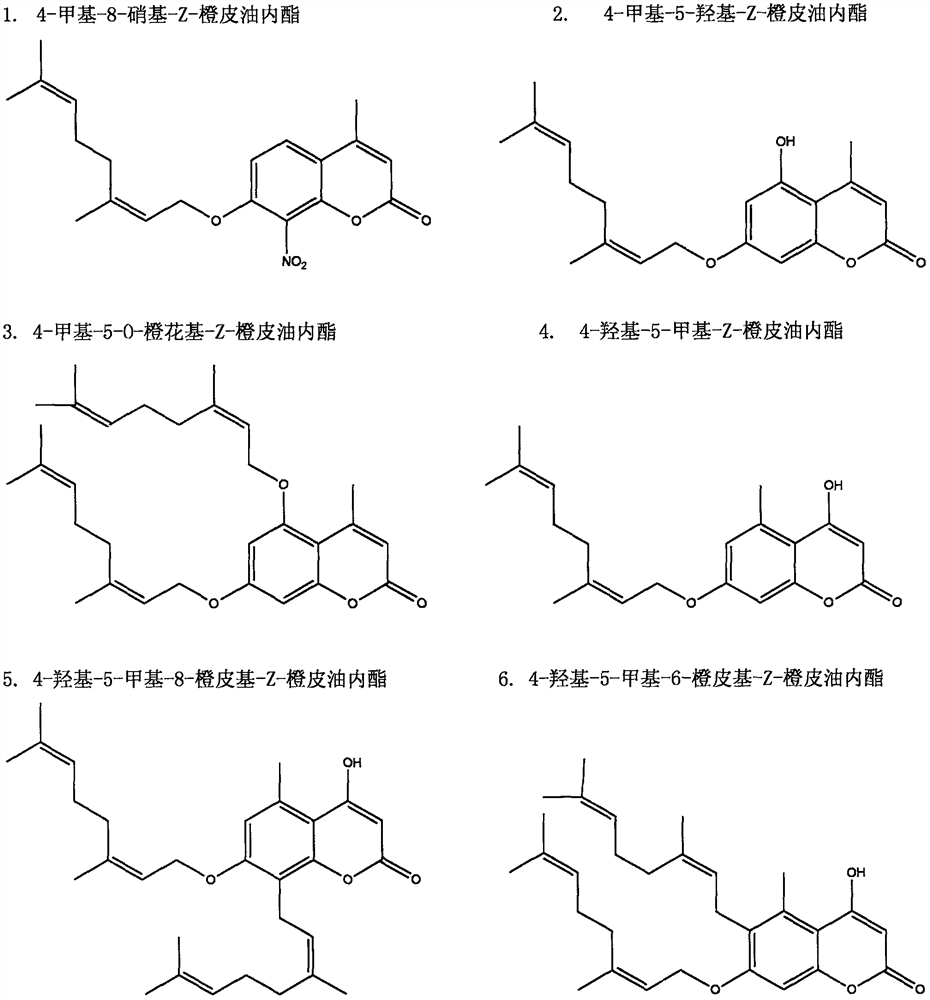
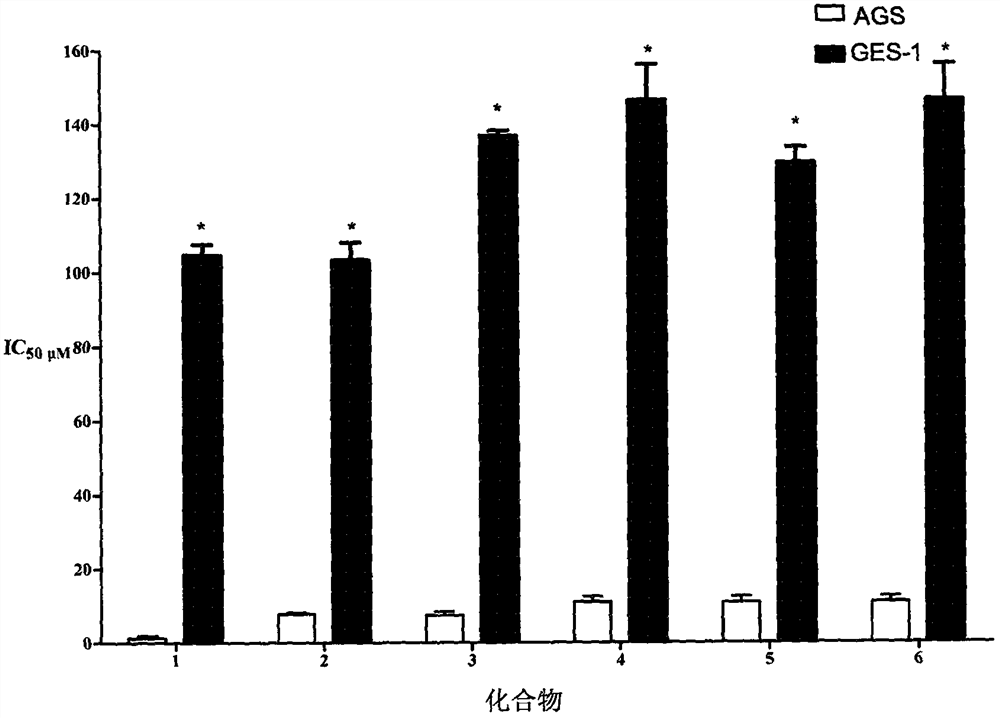
[0011] 1. Invention compound 4-methyl-8-nitro-Z-hesperidolide (1), 4-methyl-5-hydroxyl-Z-hesperidolide (2), 4-methyl- 5-O-Neryl-Z-hesperidolide (3), 4-hydroxy-5-methyl-Z-hesperidolide (4), 4-hydroxy-5-methyl-8- Synthesis experiment of hesperidyl-Z-hesperidone (5), 4-hydroxy-5-methyl-6-hesperidyl-Z-hesperidolide (6).
[0012] (1) Experimental raw material: nerolidol
[0013] (2) Method Add a certain amount of nerol, catalytic amount of pyridine (Py), and anhydrous ether into a 150ml three-necked flask, and slowly add phosphorus tribromide dropwise under conditions of ice-water bath and nitrogen protection, with the temperature controlled at 0°C Anhydrous ether solution, keep 0 ℃ reaction, prepare neryl bromide; with phloroglucinol and ethyl acetoacetate, in POCl 3 Under catalysis, the intermediate 4-methyl-5,7-dihydroxycoumarin is generated by pechmann condensation reaction; with 3,5-dihydroxytoluene and dimethylformamide (DMF) in POCl 3 Under catalysis, the intermediate com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com