Compositions and methods for treating retinal diseases
A retinal, composition-based technology for stem cell biology and regenerative medicine that addresses pluripotent cell contamination, low graft yield and efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
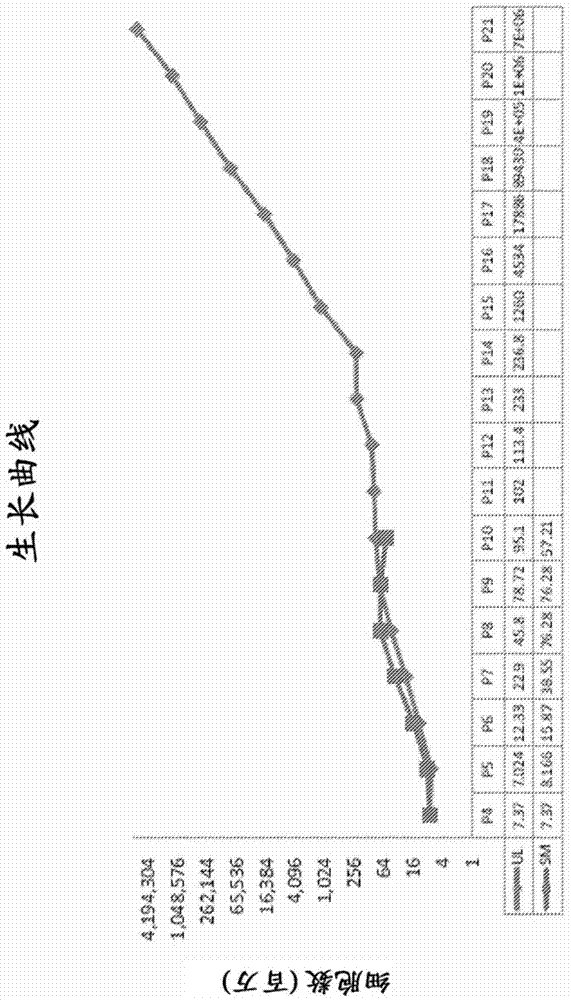
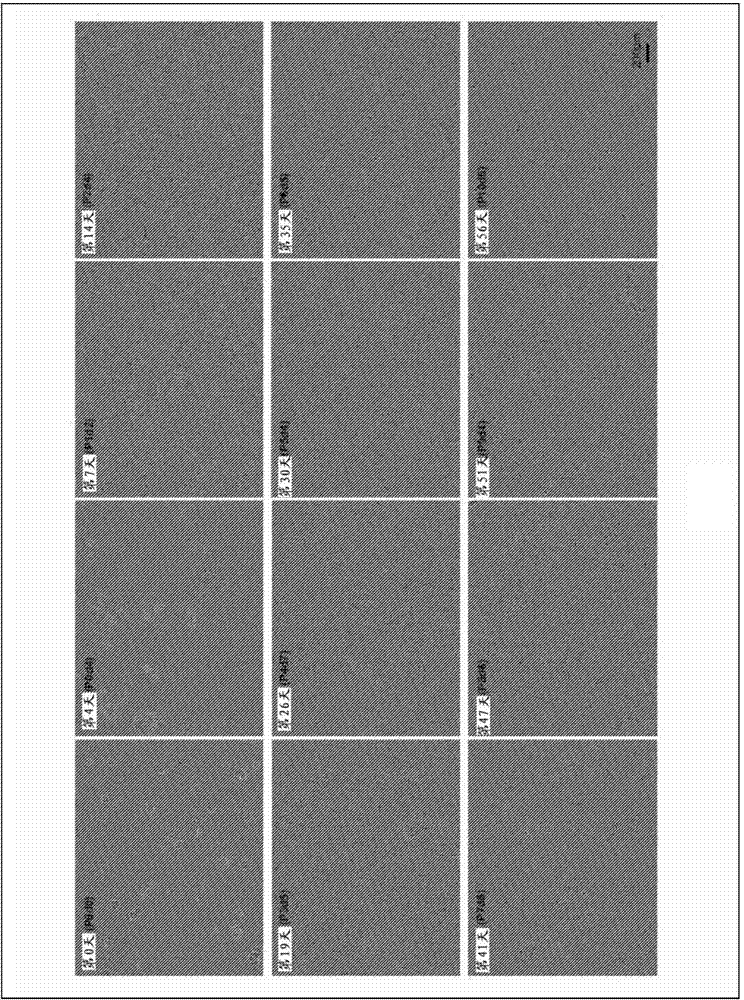
Image
Examples
Embodiment 1
[0237] Example 1: Isolation and culture of retinal progenitor cells
[0238] Whole fetal eyes of fetuses of approximately 16 to 19 weeks of gestational age (GA) were obtained from donors and placed in 15 ml tubes containing RPMI-1640 medium containing L-glutamine (BioWhittaker). Eyes were transported on ice for a period of time ranging from 4.5 hours to about 21.5 hours. At the same time, a blood sample from the donor is drawn and sent for testing for exposure to exogenous agents. Upon arrival, each eyeball is inspected to ensure that the cornea is clear and of normal shape, and the eyeball is then placed in a laminar flow clean bench under sterile conditions. Whole fetal eyes were rinsed three times with 40 ml of cold phosphate buffered saline (PBS) containing antibiotics in separate 50 ml tubes. The optic nerve and remaining mesenchymal tissue are then removed by dissection. This method was developed to avoid contamination of possible retinal isolates with unwanted cells ...
Embodiment 2
[0254] Example 2: Characterization of RPC
[0255] Immunocytochemistry
[0256] Cells were dissociated and grown on 4- or 8-well chamber slides for 1-3 days, then fixed in 4% paraformaldehyde for 15 minutes and washed 3 times in PBS. Slides were blocked for 1 hour in a solution containing 5% donkey serum and / or 0.3% Triton X-100, then washed again with PBS. It was then incubated overnight at 4°C with a panel of antibodies to detect antigens expressed by the progenitor cells. This includes anti-nestin antibody (Chemicon1:200), anti-vimentin antibody (Sigma1:200), anti-Sox2 antibody (Santa Cruz1:400), anti-SSEA-1 antibody (BD1:200), anti-GD2 antibody (Chemicon1:100 ), anti-Ki-67 antibody (BD1:200), anti-β3-tubulin antibody (Chemicon1:400), anti-GFAP antibody (Chemicon1:400), and anti-GDNF antibody (Santa Cruz1:200). Then incubated with anti-mouse Alexa546 (Invitrogen 1:400), anti-goat Alexa488 (Invitrogen 1:400), or anti-rabbit FITC (Chemicon 1:800) secondary antibody. Fluor...
Embodiment 3
[0312] Example 3: In vivo effectiveness of RPCs
[0313] RPCs for transplantation were first prepared by harvesting RPCs with TrypLE Express and centrifuging at 1000 rpm for 5 minutes to collect the RPCs. Cells were washed once in HBSS and then resuspended in cold HBSS to determine cell viability and cell number. For human transplants, use 0.5 × 10 in 100 μl HBSS 6 cells. For transplantation into rats, different doses ranging from 4000 to 75,000 cells in 2 μl of HBSS were used.
[0314] Human RPCs were transplanted as a suspension into the vitreous or subretinal space of dystrophic hooded RCS rats. Rats were given cyclosporin A and steroids to avoid xenograft rejection. Control eyes received sham injections (subretinal, intravitreal) consisting of vehicle only. Transplanted animals were functionally tested in an unrestrained wakefulness state using a commercial instrument designed to quantify optokinetic response (OR). Test the brightness threshold of a subgroup of anima...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com