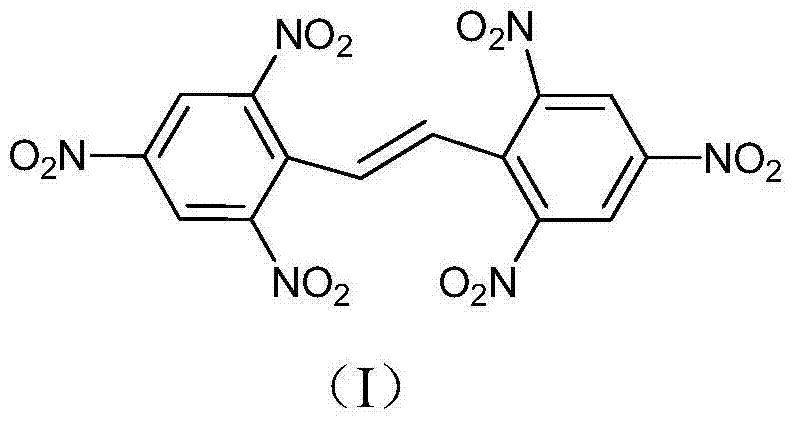
2,2',4,4',6,6'-hexanitro diphenylethylene preparation method
A technology of hexanitrodiphenylethylene and hexanitrobibenzyl, which is applied in the field of preparation of 2,2',4,4',6,6'-hexanitrodiphenylethylene, can solve the problem of benzene- Solve the problems of large loss of pyridine mixed solvent, large amount of use and loss, etc., to achieve the effect of less pyridine consumption, less loss, and reduced solvent loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
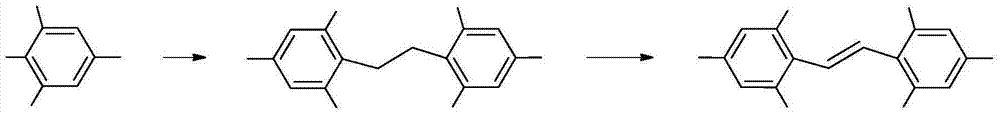
[0027] This embodiment adopts new ethanol-benzene mixed solvent to prepare hexanitrobibenzyl.
[0028] Under stirring at room temperature, add 400.0g (1.76mol) of 2,4,6-trinitrotoluene into a mixed solvent of 1200ml of benzene and 2200ml of ethanol, heat up to 55°C, add dropwise 850.0g (2.39mol) of 21% sodium hypochlorite aqueous solution, after the dropwise addition, react at a temperature of 55°C for 45min, cool down to 20°C, filter the reaction solution, collect the filtrate to obtain 4000ml, and wash the filter cake with water, rinse with ethanol and dry to obtain hexanitrobibenzyl 299.2 g, yield 75.2%, DSC: 223.6°C.
Embodiment 2
[0030] In this example, the filtrate in Example 1 is recycled as a reaction solvent to prepare hexanitrobibenzyl.
[0031] Take 4000ml of the filtrate of Example 1, add 2000ml of saturated saline, shake, let it stand for 45min, separate the liquid, collect the upper layer of benzene liquid to obtain 1000ml, add 300ml of benzene and 2200ml of ethanol, and use the resulting mixture as a reaction solvent.
[0032] Under stirring at room temperature, add 400.0g (1.76mol) 2,4,6-trinitrotoluene to the mixed solvent prepared above, heat up to 55°C, and drop 850.0g (2.39mol) with a mass concentration of 21% The sodium hypochlorite aqueous solution, after the dropwise addition, was reacted at a temperature of 55°C for 45min, cooled to 20°C, the reaction solution was filtered, the filtrate was collected, and the filter cake was washed with water, rinsed with ethanol and dried to obtain 294.0 g of hexanitrobibenzyl, with a yield of 73.9 %, DSC: 214.3°C.
Embodiment 3
[0034] This example uses a new dichloroethane-pyridine mixed solvent to prepare 2,2',4,4',6,6'-hexanitrodiphenylethylene.
[0035] Under stirring at room temperature, add 240.0g (0.53mol) of hexanitrobibenzyl into a mixed solvent of 1600ml of dichloroethane and 300ml of pyridine, heat up to 70°C, add 160.0g (1.0mol) of liquid bromine dropwise, and add dropwise After completion, react at a temperature of 80°C for 3 hours, lower the temperature to 20°C, filter the reaction solution, collect the filtrate to obtain 1700ml, wash the filter cake with water, rinse with acetone and dry to obtain 2,2',4,4',6,6' - 233.0 g of hexanitrodiphenylethylene, yield 97.5%, DSC: 325.5°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com