A kind of bishydrazide compound and preparation method thereof
A compound, bishydrazide technology, applied in the field of inhibitors and its preparation, can solve problems such as poor lethality, and achieve the effect of wide application range and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
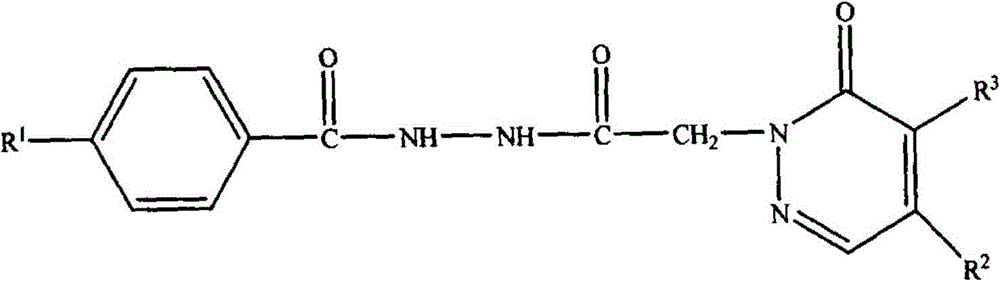
[0041] A bishydrazide compound, the compound molecular formula is as follows:
[0042]
[0043] Among them, R 1 is methyl, R 2 is morpholinyl, R 3 For chlorine, the synthetic method of this bishydrazide compound comprises the following steps:
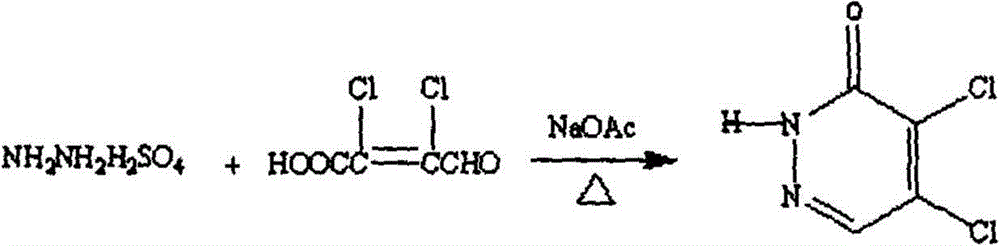
[0044] (1) Preparation of 4,5-dichloro-3(2H)pyridazinone
[0045]
[0046] In a 250mL three-necked flask, add 50g of dichlorobutenalic acid and a small amount of water, stir to make an aqueous solution, then add 39g of hydrazine sulfate and 38.2g of sodium acetate, heat to 80-100°C, and react for 2 hours. After complete reaction, cool down, pour the reaction solution into cold water, a large amount of light yellow precipitate appears, filter it with suction, and dry it. The obtained product was recrystallized with absolute ethanol, and the yield was 89.5%.
[0047] (2) Synthesis of 4-methyl-benzohydrazide
[0048]
[0049] In a 250ml three-necked flask, add 70ml (0.485mol) of 4-methyl-ethylbenzoate (ie ethyl p-toluate) an...
Embodiment 2
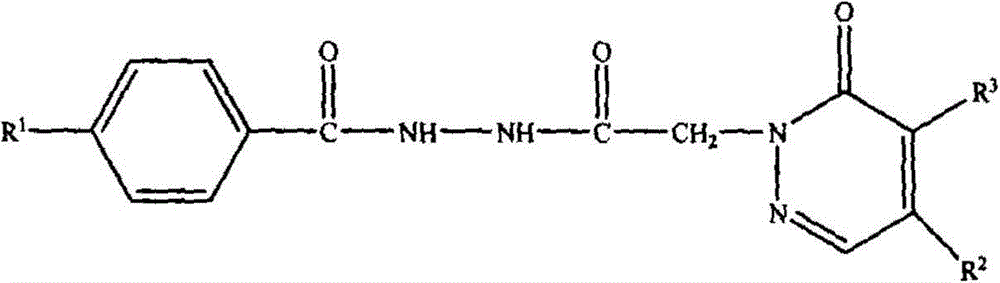
[0067] A bishydrazide compound, the compound molecular formula is as follows:
[0068]
[0069] Among them, R 1 is methyl, R 2 is piperidinyl, R 3 For chlorine, the synthetic method of this bishydrazide compound comprises the following steps:
[0070] (1) Preparation of 4,5-dichloro-3(2H)pyridazinone
[0071]
[0072] In a 250mL three-necked flask, add 50g of dichlorobutenalic acid and a small amount of water, stir to make an aqueous solution, then add 39g of hydrazine sulfate and 38.2g of sodium acetate, heat to 80-100°C, and react for 2 hours. After complete reaction, cool down, pour the reaction solution into cold water, a large amount of light yellow precipitate appears, filter it with suction, and dry it. The obtained product was recrystallized with absolute ethanol, and the yield was 89.5%.
[0073] (2) Synthesis of 4-methyl-benzohydrazide
[0074]
[0075] In a 250ml three-neck flask, add 70ml (0.485mol) of 4-methyl-benzoic acid ethyl ester and 51.5ml (0....
Embodiment 3
[0093] A bishydrazide compound, the compound molecular formula is as follows:
[0094]
[0095] Among them, R 1 is methyl, R 2 is dimethylamino, R 3 For chlorine, the synthetic method of this bishydrazide compound comprises the following steps:
[0096] (1) Preparation of 4,5-dichloro-3(2H)pyridazinone
[0097]
[0098] In a 250mL three-necked flask, add 50g of dichlorobutenalic acid and a small amount of water, stir to make an aqueous solution, then add 39g of hydrazine sulfate and 38.2g of sodium acetate, heat to 80-100°C, and react for 2 hours. After complete reaction, cool down, pour the reaction solution into cold water, a large amount of light yellow precipitate appears, filter it with suction, and dry it. The obtained product was recrystallized with absolute ethanol, and the yield was 89.5%.
[0099] (2) Synthesis of 4-methyl-benzohydrazide
[0100]
[0101] In a 250ml three-neck flask, add 70ml (0.485mol) of 4-methyl-benzoic acid ethyl ester and 51.5ml (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com