Use of oligosaccharide compounds for the prevention and treatment of pathological scars
A compound and pathological technology, applied in skin care preparations, medical preparations containing active ingredients, cosmetics, etc., can solve the problem of wounds that cannot produce hypertrophic scars
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
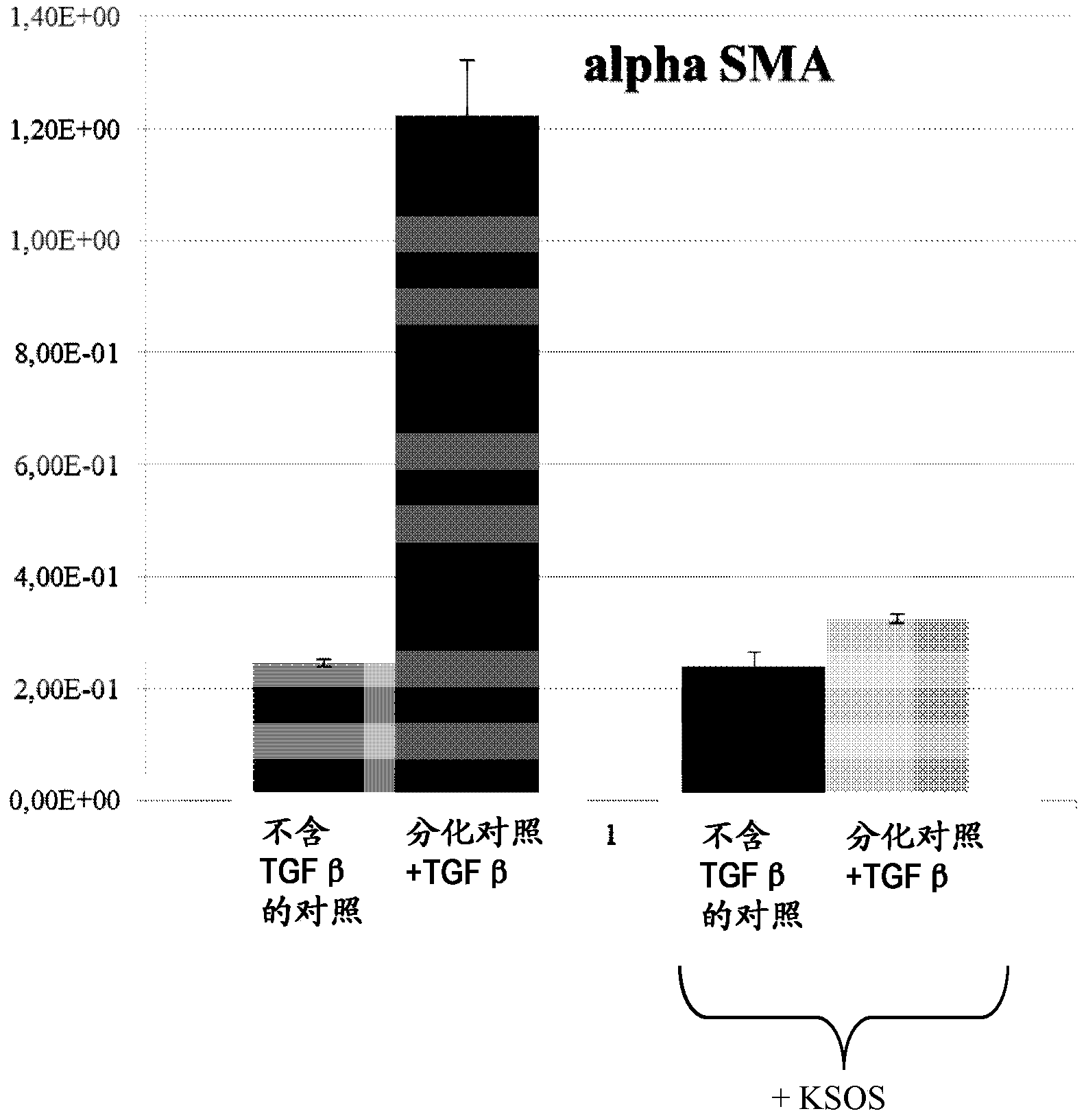
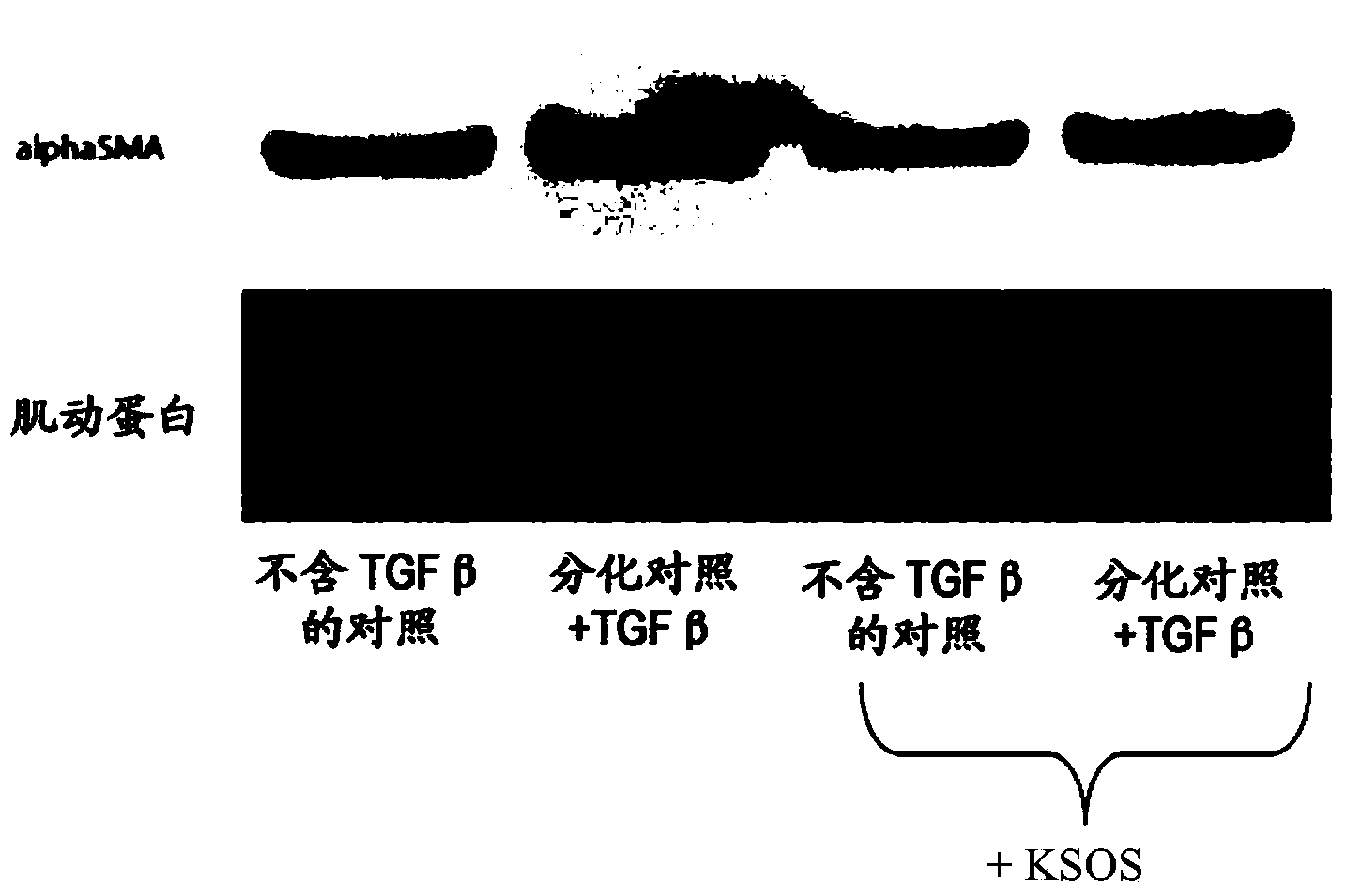
[0131] Example 1: Effects of Sucrose Octasulfate Potassium Salt (KSOS) on Myofibroblast Differentiation proof of
[0132] 1. Culture of normal human dermal fibroblasts (NHDF)
[0133] DMEM / F12 medium (marketed by Invitrogen) supplemented with 10% fetal bovine serum (marketed by Invitrogen), 5 μg / ml of insulin (marketed by Promokine) and 1 ng / ml of bFGF (marketed by Promokine) ) cultures of normal human dermal fibroblasts (NHDF).
[0134] 2. induced differentiation
[0135] Then, the fibroblasts are induced to differentiate into myofibroblasts.
[0136] To this end, NHDF cultures were placed on P100 dishes coated with 5 μg / ml collagen in DMEM / F12 medium supplemented with 10% fetal bovine serum and supplemented with 10 ng / ml ml of TGF-β (marketed by Promocell), which is a growth factor that controls cell proliferation and differentiation (differentiation control in Figure 1).
[0137] In addition, cultures in DMEM / F12 supplemented with 10% fetal calf serum but withou...
Embodiment 2
[0148] Example 2: Effect of Potassium Sucrose Octasulfate (KSOS) on Collagen Lattice Contraction proof of
[0149] 1. Preparation of Collagen Lattice
[0150] By inoculating 0.8 × 10 gels with type I collagen at a final concentration of 1.3 mg / ml 6 normal human dermal fibroblasts (NHDF) to obtain collagen lattices.
[0151] 2. Demonstration of the effect of potassium sucrose octasulfate (KSOS) on collagen lattice contraction
[0152] A dressing containing 7.5% potassium sucrose octasulfate (sold under the trade name Urgotul Start) was applied to the surface of the collagen lattice.
[0153] To evaluate the normal contraction of collagen gels induced by inoculated NHDF, a control series without dressing was performed.
[0154] Also, in order to visualize the strong contraction induced by NHDF of highly differentiated myofibroblastic cells, a positive control was performed by adding TGF-β at a concentration of 10 ng / ml.
[0155] The incubation was maintained up to 7 ...
Embodiment 3
[0163] A solvent-based filmogel-type formulation comprising a synthetic polysulfated oligosaccharide according to the invention was prepared having the following composition:
[0164] components
[0165] Dilute the nitrocellulose in a mixture of ethyl acetate / absolute ethanol. Castor oil, UV screener and KSOS are then added until dissolved to obtain a filmogel type composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com