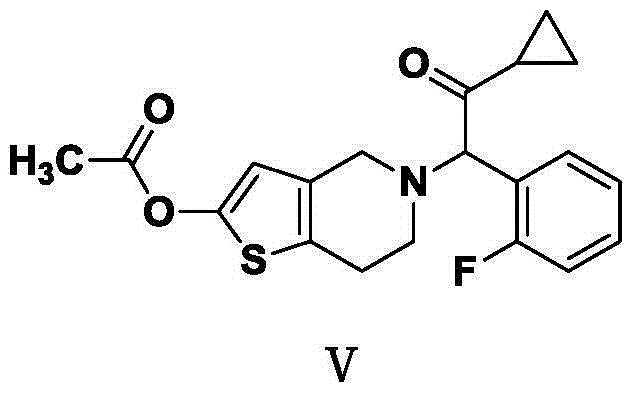
Hydrogenated pyridine derivatives and preparation method thereof
A technology for hydrogenating pyridine and derivatives, applied in the direction of organic chemistry, etc., to achieve the effect of speeding up the reaction, reducing the generation of by-products, and mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
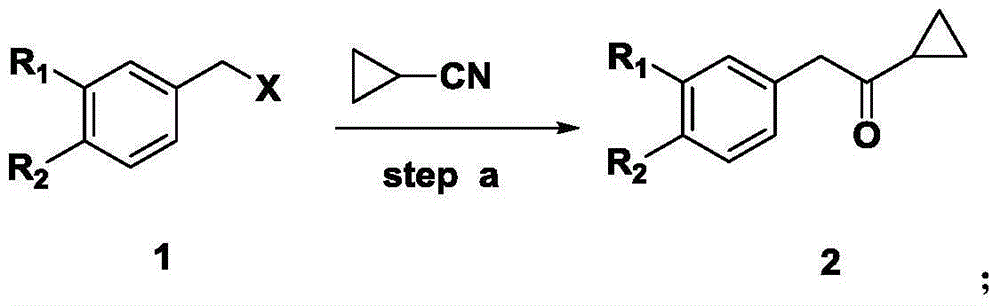
[0042] Compound 2: Preparation of 2-phenyl-1-cyclopropylethanone
[0043] in N 2 Under protection, add about 1-2ml of benzyl chloride (38g, 0.3mol) diluted with 114ml of tetrahydrofuran dropwise into a reaction flask containing 114ml of tetrahydrofuran, magnesium powder (8.6g, 0.36mol) and a few grains of iodine, and heat the reaction slightly. System, after the reaction is initiated and the brown color fades, slowly add the remaining benzyl bromide-tetrahydrofuran solution dropwise under temperature control at 25-30°C. Add dropwise to 114ml tetrahydrofuran diluted cyclopropylnitrile (24.1g, 0.36mol) at ~10°C. After the dropwise addition, keep the reaction at room temperature for 8 hours, stop the reaction, add 200ml ether to the reaction system, and then 25~ Add 100ml of water dropwise to the reaction solution at 30°C, then adjust the pH of the system to <4 with 2N hydrochloric acid at 25-30°C, separate the water phase, wash the organic phase with 150ml of 5% sodium bicarbon...
Embodiment 2
[0045] Compound 2: Preparation of 2-phenyl-1-cyclopropylethanone
[0046] in N 2Under protection, add about 1-2ml of benzyl bromide (30g, 0.17mol) diluted with 150ml of ether to the reaction flask with 150ml of ether, magnesium powder (6.3g, 0.26mol) and a few grains of iodine, and heat the reaction slightly. System, after the reaction is initiated and the brown color subsides, slowly add the remaining benzyl bromide-ether solution dropwise at a temperature of 15-20°C. After the dropwise addition, keep stirring at 15-20°C for 1 hour, and then add the Grignard reagent obtained above to - Add dropwise to 150ml of cyclopropylnitrile (17.6g, 0.26mol) diluted with ether at 5-0°C. After the dropwise addition, keep the reaction at room temperature for 8 hours, stop the reaction, and drop into the reaction solution at 25-30°C Add 100ml of water, adjust the pH of the system to <4 with 2N hydrochloric acid at 25-30°C, remove the aqueous phase, wash the organic phase with 120ml of 5% so...
Embodiment 3
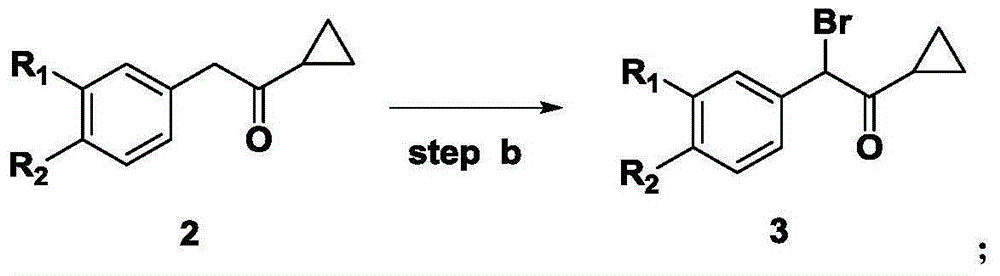
[0048] Compound 3: Preparation of 2-phenyl-2-bromo-1-cyclopropylethanone
[0049] Add 2-phenyl-1-cyclopropylethanone (39.3g, 0.25mol), PTSA (3.9g), 120ml methanol, NBS (48.0g, 0.27mol) to the reaction flask, and react at 40-45°C for 5 hour, after stopping the reaction, add 120ml ethyl acetate and 120ml5% sodium bicarbonate solution to the reaction system, separate the water phase, and then wash the organic phase with 120ml water, evaporate the solvent to obtain 54g yellow liquid, yield 91.6% , HPLC: 94.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com