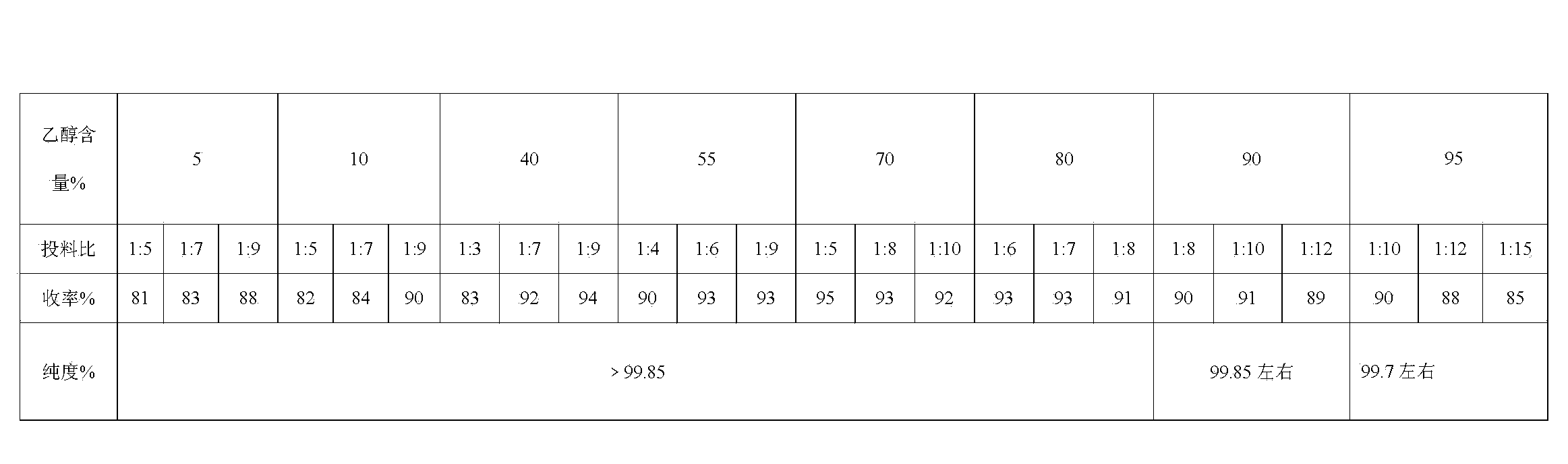Preparation method for high-purity sulpiride or optical isomers thereof
An optical isomer, high-purity technology, applied in the field of medicine, can solve the problems of large dosage, high production cost, low solubility, etc., and achieve the effects of reducing the amount of related substances, reducing production costs, and reducing the material feeding ratio.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
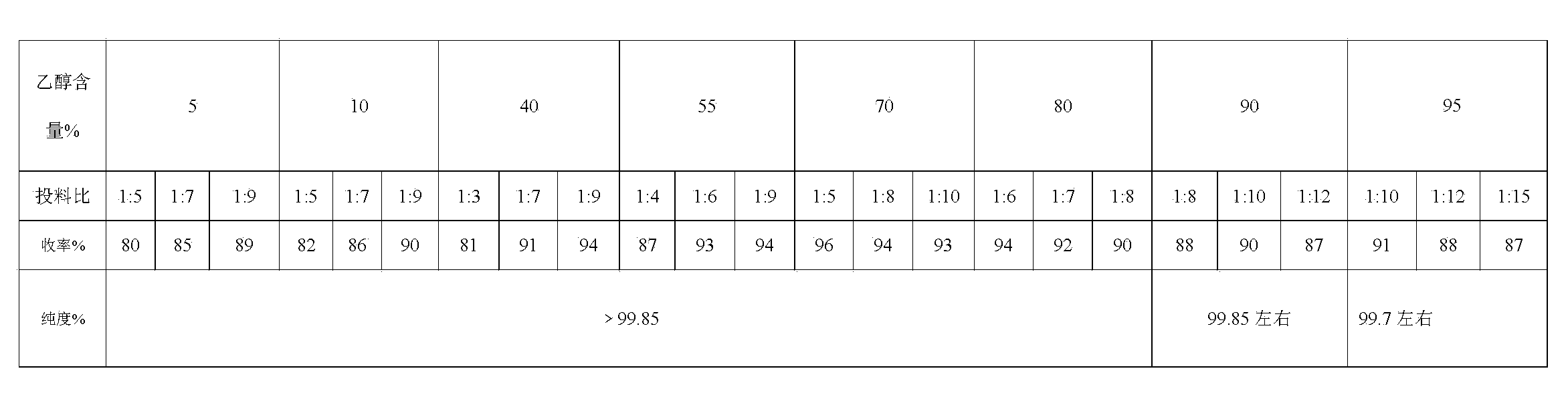
Image
Examples
Embodiment 1
[0040] Add 160g of 90% ethanol to the reaction flask, add 20g of the crude product of sulpiride, heat and stir under the protection of nitrogen until the crude product is completely dissolved, cool slightly, add 0.8g of activated carbon, stir under reflux for 5 minutes, filter while it is hot, and cool the filtrate to 0-5 Stir at ℃ for 1 hour, filter, wash with 90% ethanol, and dry at 70℃ for 2 hours. The product was obtained with a yield of 90.3%. Related substances are less than 0.15%.
Embodiment 2
[0042] Add 100g of 70% ethanol to the reaction flask, add 20g of the crude product of sulpiride, under the protection of nitrogen, heat and stir until the crude product is completely dissolved, filter while it is hot, cool the filtrate to 0-5°C and stir for 0.5 hours, filter, wash with 70% ethanol, Dry at 70°C for 2 hours. The product was obtained with a yield of 95.4%. Related substances are less than 0.1%.
Embodiment 3
[0044] Add 70g of 40% ethanol to the reaction flask, add 10g of crude sulpiride, heat and stir until the crude product is completely dissolved, cool slightly, add 0.1g of activated carbon, stir at reflux for 15 minutes, filter while it is hot, cool the filtrate to 0-5°C and stir for 1 hour , filtered, washed with 40% ethanol, and dried at 70°C for 2 hours. The product was obtained with a yield of 92.2%. Related substances are less than 0.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com