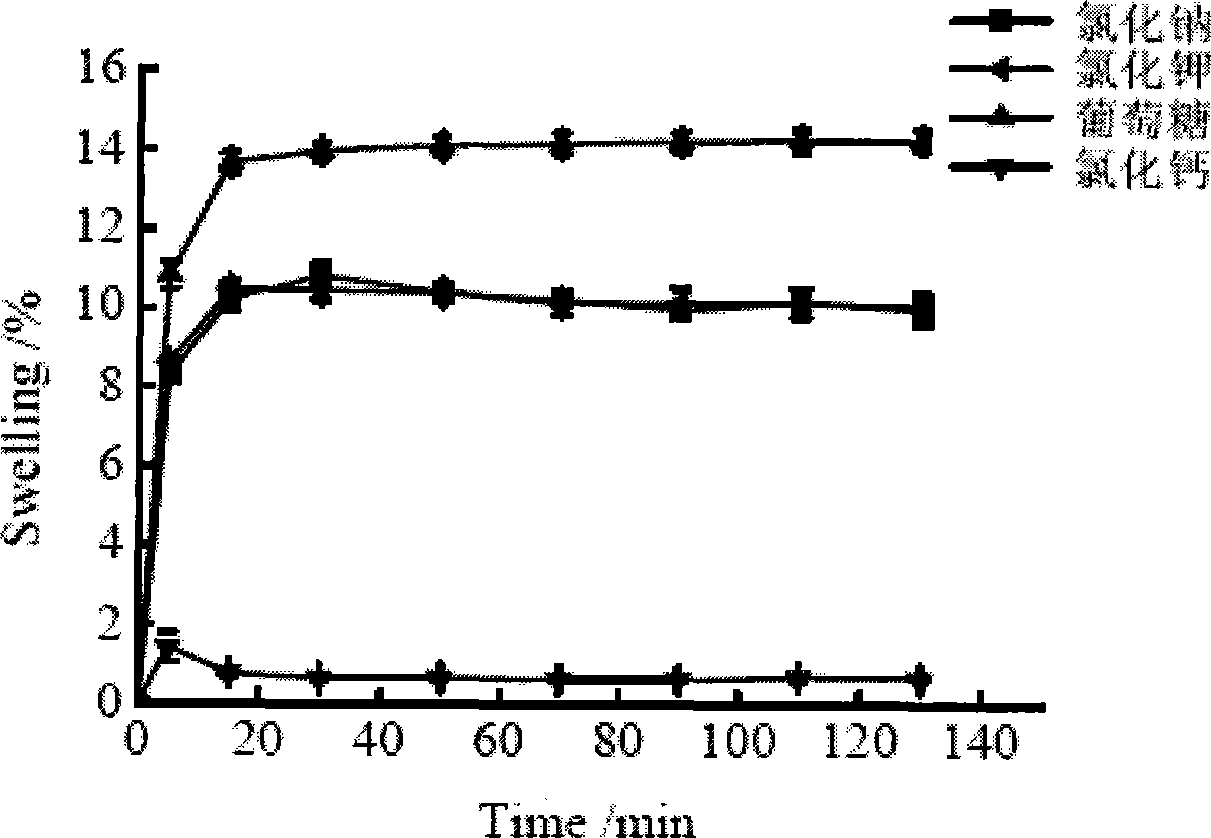
Detection method of protein content in drug gel
A technology of protein content and detection method, which is applied in the field of detection of protein content in pharmaceutical gels, can solve the problems of inaccurate quantification, difficulty in controlling the release amount, and large differences in results, etc., and achieves easy to master operation, reasonable design, and repeatable strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Taking the detection of the rh-bFGF content in the recombinant human basic fibroblast growth factor (rh-bFGF) gel as an example.
[0035] bFGF gel is the latest new drug product of Beijing Shuanglu Pharmaceutical Co., Ltd. for the treatment of tissue wound healing. Each rh-bFGF gel for external use contains 5g rh-bFGF gel, and each 1g contains 5000IU rh-bFGF.
[0036] 1. The process prescription is:
[0037]
[0038] 2. Preparation process:
[0039] According to the calculated amount, take glycine and ethyl 4-hydroxybenzoate by weighing respectively and put into (two-thirds of the total amount) water for injection to prepare the aqueous solution of glycine and ethyl 4-hydroxybenzoate. Heat to dissolve and stir well. Take a quarter of it and autoclave it for later use (liquid A).
[0040] Weigh the carbomer according to the calculated amount and dissolve it in the above aqueous solution to make a carbomer gel, which is autoclaved for future use (liquid B).
[0041]...
Embodiment 2
[0053] Determination of the content of drug protein rh-bFGF in bFGF gel
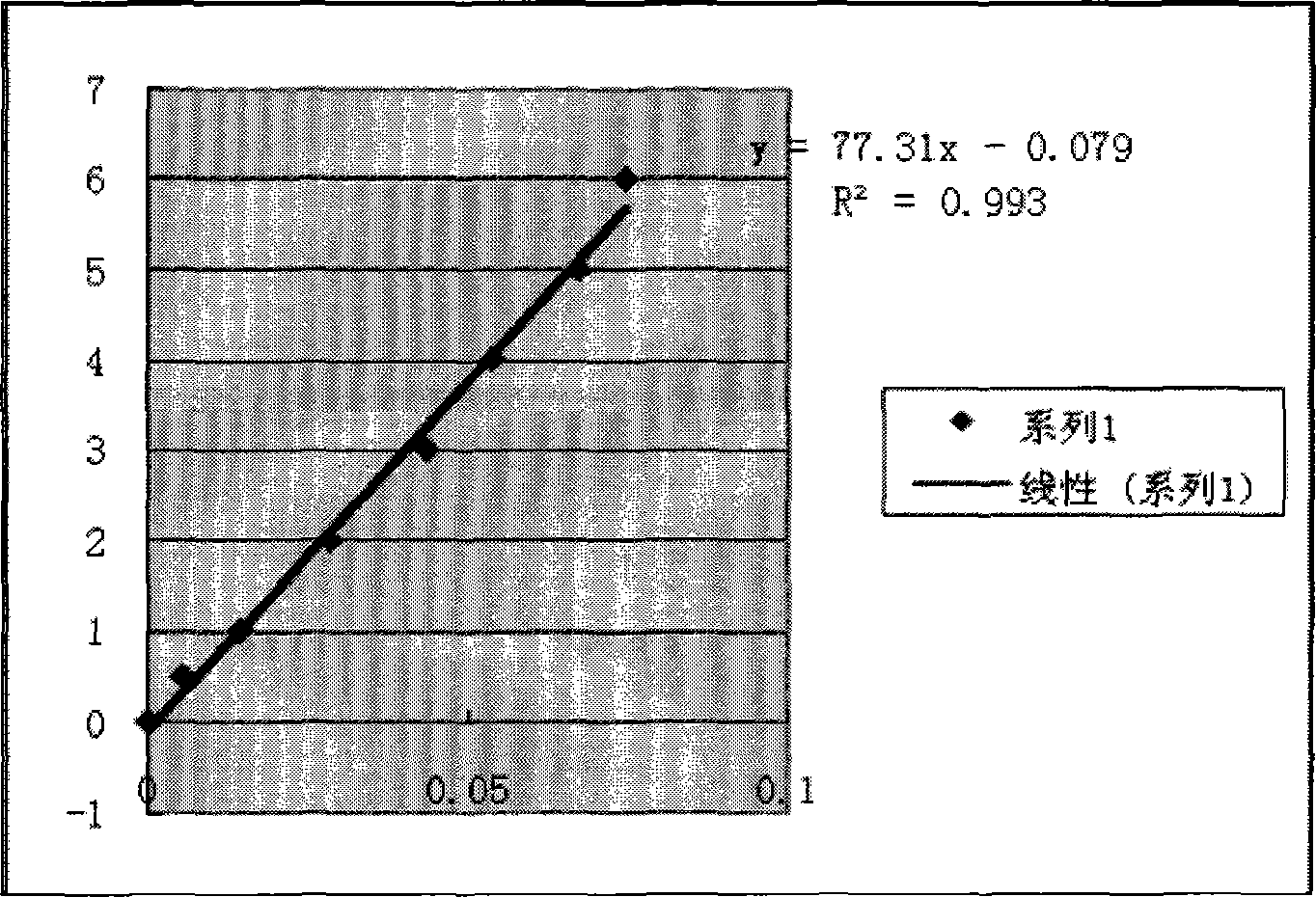
[0054] 1. Determination of rh-bFGF microplate reader method detection standard working curve
[0055] Accurately measure 10mL of rh-bFGF solution with a concentration of 12.5mg / ml, add double distilled water to make the volume to 250mL, dilute the rh-bFGF standard to 50μg / ml, and accurately measure 0, 10, 20, 40, 60, 80, 100 and 120 μl were sucked into 1.5ml eppendorf tubes, and the gel blank was added to 800 μl, and 200 μl Bradford working solution was added to each tube (preparation of Bradford working solution: take 25ml 95% ethanol, 50ml 85% phosphoric acid, 50mg Coomassie brilliant blue G- 250 to mix evenly, add distilled water to dilute to 500ml), develop color for 5 minutes, pipette 250μl into a 96-well plate, and scan the absorbance value at 540nm with a microplate reader.
[0056] Take the rh-bFGF standard substance concentration as the ordinate and the light absorption value at 540 nm as the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com