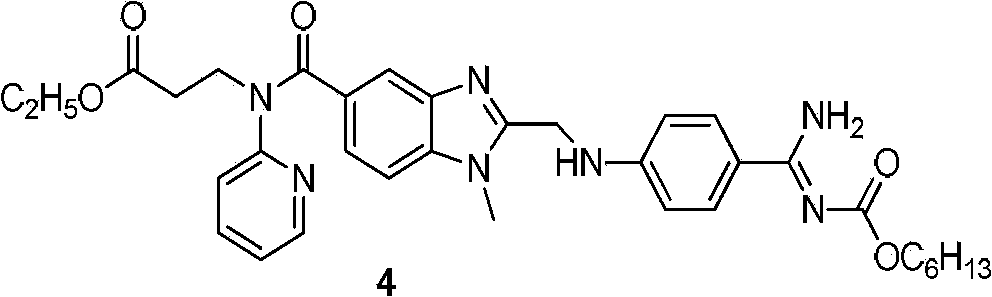
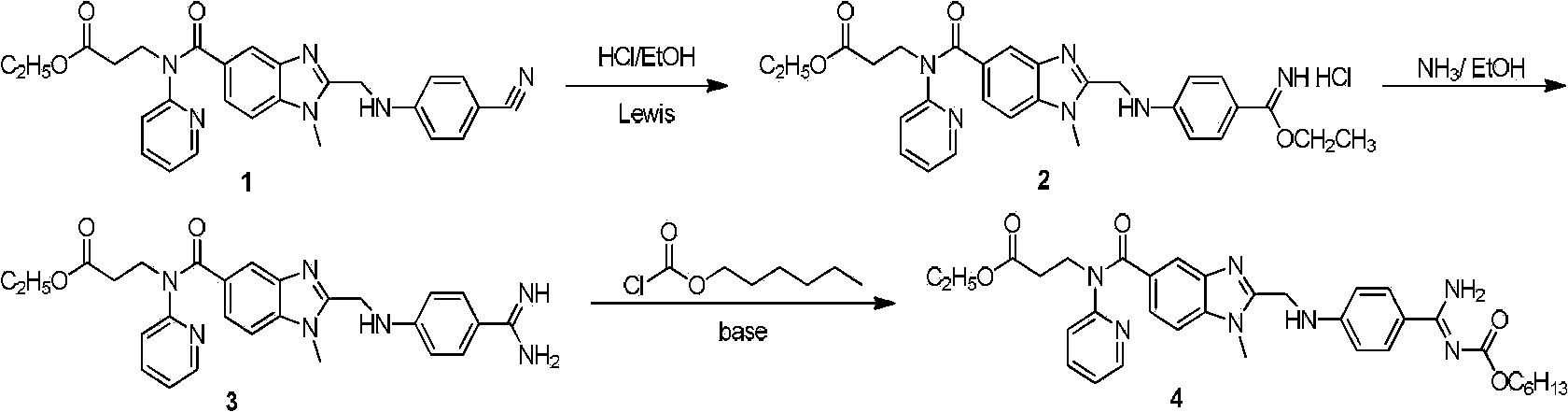
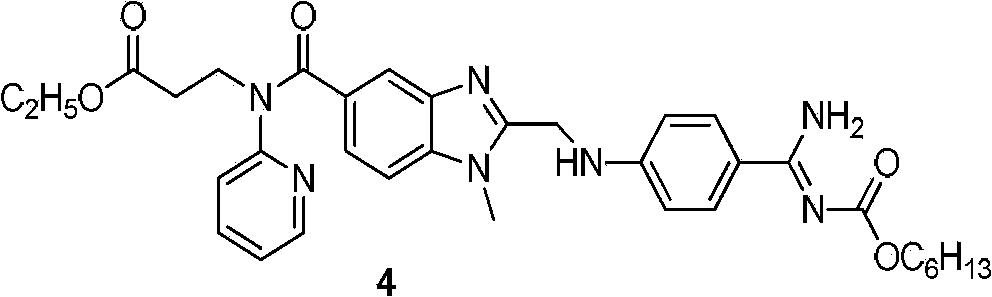
Dabigatran preparation method
A technology of dabigatran etexilate and molar ratio, applied in the field of preparation of dabigatran etexilate, can solve problems such as low yield, long reaction time, and incomplete reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: The ratio of the amount of Lewis acid to compound 1 is 0.4:1
[0020] Dissolve 80g (0.166mol) of the compound of formula 1 in freshly prepared saturated hydrochloric acid ethanol solution (1700ml), stir and dissolve at room temperature until the solution turns yellow-green and clear, then add 9.03g (0.066mol) zinc chloride solid. A white solid was precipitated after 3 hours of reaction, traced by thin-layer chromatography, and the reaction was complete after 10 hours. Concentrate with rotary evaporation at a heating temperature of 45-50° C., concentrate to 300 ml, add 1400 ml of ethyl acetate, stir for 3 hours, filter, and dry in air to obtain 115 g of a solid product of formula 2.
[0021] Dissolve the above solid with 1500ml of absolute ethanol, stir evenly and turn into pink turbidity, and slowly add 248ml of ammonia water dropwise in an ice-water bath. During the dropwise addition, the color of the reaction solution changed from pink turbidity to a co...
Embodiment 2
[0022] Embodiment 2: the ratio of the consumption of Lewis acid and formula 1 compound 0.2:1
[0023] Dissolve 80g (0.166mol) of the compound of formula 1 in freshly prepared saturated hydrochloric acid ethanol solution (1700ml), stir and dissolve at room temperature until the solution turns yellow-green and clear, then add 4.52g (0.033mol) zinc chloride solid. A white solid was precipitated after 3 hours of reaction, traced by thin-layer chromatography, and the reaction was complete after 10 hours. Concentrate by rotary evaporation and heating at a heating temperature of 45-50° C., concentrate to 300 ml, add 1400 ml of ethyl acetate, stir for 3 hours, filter, and dry in air to obtain 120 g of a solid product of formula 2.
[0024] Dissolve the above solid with 1565ml of absolute ethanol, stir evenly and turn into pink turbidity, and slowly add 258ml of ammonia water dropwise in an ice-water bath. During the dropwise addition, the color of the reaction solution changed from p...
Embodiment 3
[0025] Embodiment 3: the ratio of the consumption of Lewis acid and formula 1 compound 1.5:1
[0026] Dissolve 80g (0.166mol) of the compound of formula 1 in freshly prepared saturated hydrochloric acid ethanol solution (1700ml), stir and dissolve at room temperature until the solution turns yellow-green and clear, then add 33.94g (0.249mol) zinc chloride solid. A white solid was precipitated after 3 hours of reaction, traced by thin-layer chromatography, and the reaction was complete after 10 hours. Concentrate by rotary evaporation and heating at a heating temperature of 45-50°C, concentrate to 300ml, add 1400ml of ethyl acetate, stir for 3 hours, filter, and dry in air to obtain 118g of the compound of formula 2.
[0027] Dissolve the above solid with 1550ml of absolute ethanol, stir evenly and turn into pink turbidity, and slowly add 254ml of ammonia water dropwise in an ice-water bath. During the dropwise addition, the color of the reaction solution changed from pink tur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com