Synthesis of laminine Schiff base mononuclear complex with anticancer activity, and pharmaceutical composition thereof
A technology of lamininic Schiff base and lamininic acid, which is applied in the field of drug synthesis, can solve the problems such as the shortage of money, the inability to meet the diversity of cancers, and the development trend of high incidence.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
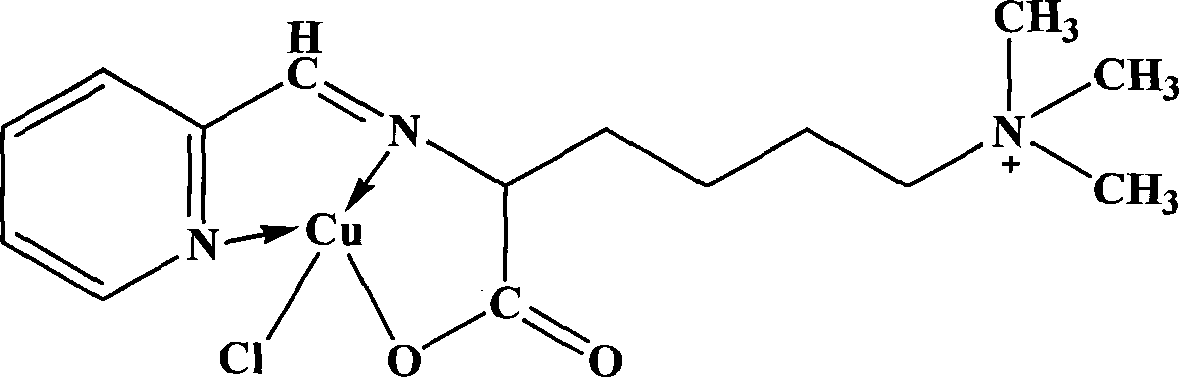
[0057] The synthetic steps of lamininic acid 2-pyridine formaldehyde Schiff base copper mononuclear complex are:
[0058] Step A: 40mmol (4.28g) of 2-pyridinecarbaldehyde was diluted with 10mL of absolute ethanol, and slowly added dropwise to 40mL of ethanol solution in which 40mmol (7.56g) of laminacin was dissolved. After the dropwise addition, the temperature was slowly raised to 70° C., and the reaction was continued for 4 hours. The solution was evaporated to about 5 mL remaining, and 20 mL of ice ethanol was added, and a large amount of precipitate formed. The solution was filtered, washed with ice ethanol and diethyl ether for several times, and dried in vacuo to obtain the white target product which was 2-pyridinecarbaldehyde Schiff base of laminacin.
[0059] Step B: 40mmol (7.56g) CuCl 2 ·3H 2 O was diluted with 10 mL of absolute ethanol, and slowly added dropwise to 40 mL of ethanol solution in which 40 mmol (11.08 g) of laminacin 2-pyridine formaldehyde Schiff b...
Embodiment 2
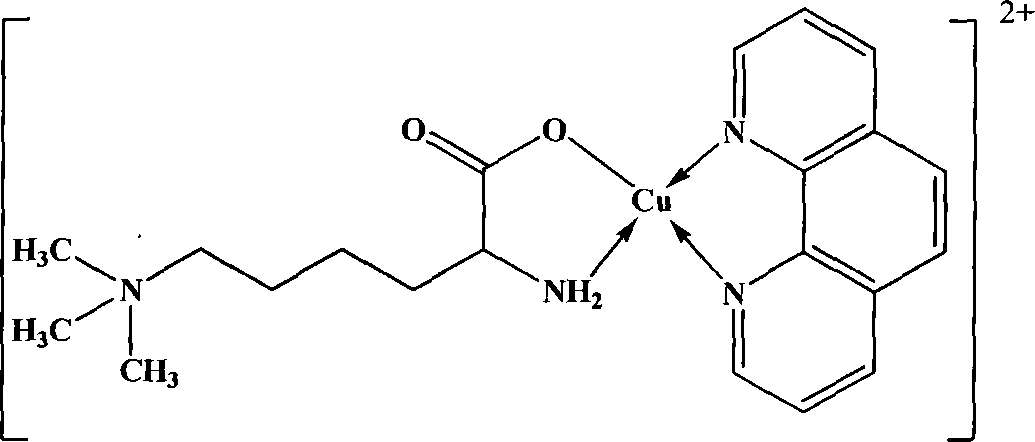
[0065] The synthetic steps of lamininine o-phenanthroline copper mononuclear complex are:
[0066] Dilute 40mmol (7.92g) of phenanthroline with 10mL of absolute ethanol, and slowly add dropwise to 40mL of aqueous solution in which 40mmol (10.04g) of the copper complex of lamininate is dissolved. After the dropwise addition, the temperature was slowly raised to 60° C., and the reaction was continued for 3 hours. The solution was evaporated to about 5 mL remaining, and a large amount of precipitate formed. The solution was filtered, washed several times with glacial ethanol and diethyl ether, and dried in vacuum to obtain the blue target product. figure 2 It is the structural formula of the laminadinine o-phenanthroline copper mononuclear complex synthesized in Example 2 of the present invention. See Table 3 and Table 4 for IR peak positions and element trace analysis results. The target product is very stable at room temperature, and is insoluble in carbon tetrachloride, ch...
Embodiment 3
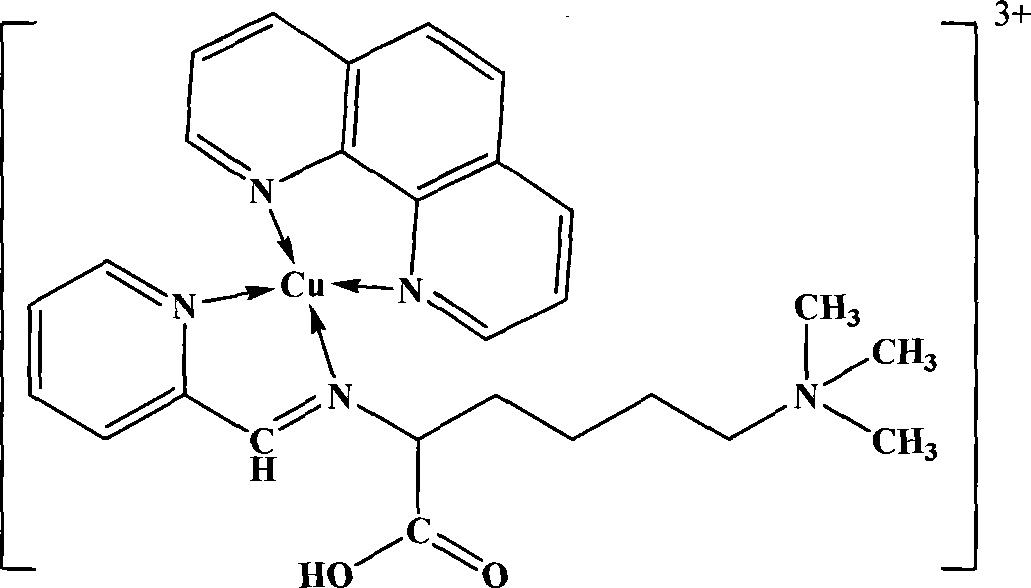
[0072] Synthesis of copper mononuclear complex of 2-pyridineformaldehyde Schiff base o-phenanthroline of lamininic acid:
[0073] Step A: According to the method shown in step A of the preparation method (1), synthesize lamininic acid 2-pyridine formaldehyde Schiff base.
[0074] Step B: 20mmol (3.78g) CuCl 2 ·3H 2 O was diluted with 10mL of absolute ethanol, and added dropwise to 10mL of absolute ethanol solution in which 40mmol (7.92g) of phenanthroline was dissolved, and after reacting for one hour, slowly added dropwise into 2 - 40 mL ethanol solution of pyridine formaldehyde Schiff base. After the dropwise addition, the temperature was slowly raised to 70° C., and the reaction was continued for 4 hours. The solution was evaporated to about 10 mL remaining, and 20 mL of ice ethanol was added, and a large amount of precipitate was formed. The solution was filtered, washed several times with glacial ethanol and diethyl ether, and dried in vacuum to obtain the blue target...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com