Application of ginsenoside F1 in preparation of anti-atherosclerosis medicament
A technology for atherosclerosis and ginsenosides, applied in the field of medicine, can solve the problems of complex formation mechanism and unsatisfactory curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
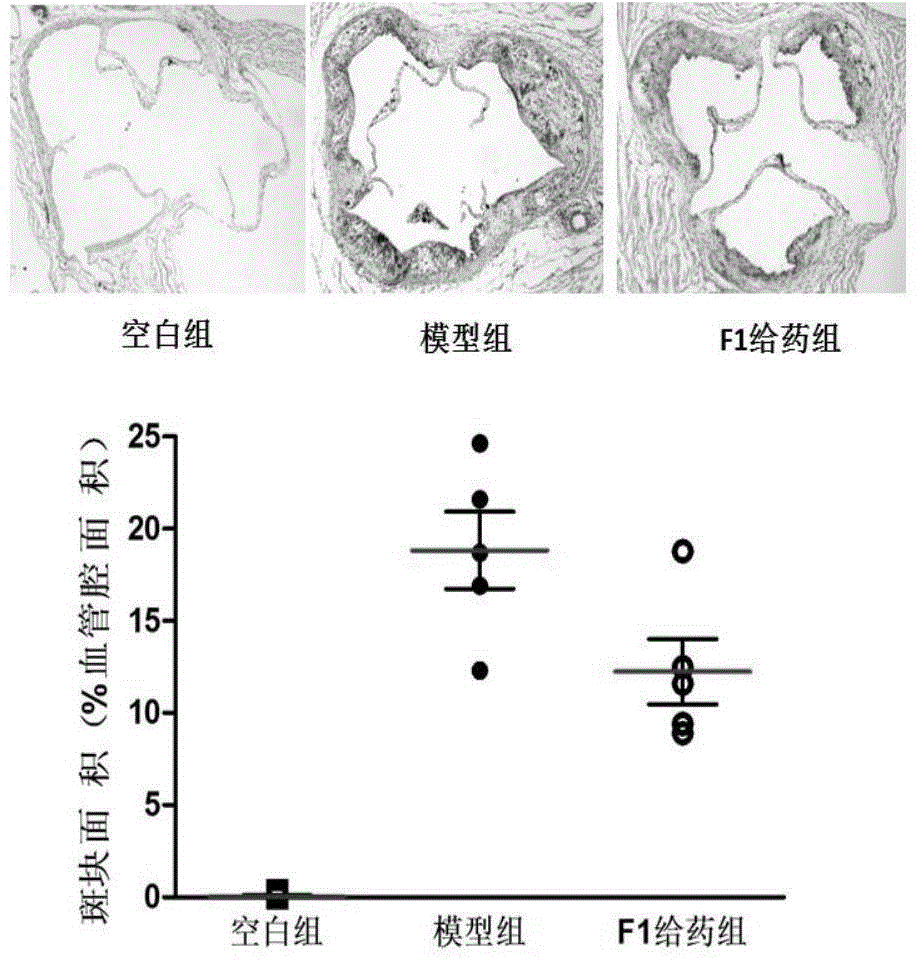
[0017] Example 1: The effect of ginsenoside F1 on the formation of atherosclerotic plaques in the aortic arch of ApoE- / - mice.
[0018] Apolipoprotein E gene knockout ApoE- / - mice were induced with high-fat diet for 8 weeks to establish atherosclerotic plaque model, C57 mice were used as blank control, and the administration group was given 50 mg / kg ginsenoside F1 every day. After the experiment, they were sacrificed by dislocation, fixed by perfusion with 4% paraformaldehyde, and the aortic arch and nearby tissues were collected for OTC embedding and stored in a -80°C refrigerator. Frozen sections were kept from the time when three complete aortic valves appeared, 80 pieces were reserved for each, and every other 4 pieces were picked for staining (16 pieces in total) and stained with 0.5% oil red, recovered with hematoxylin, and washed with water to return to blue. The slides were sealed with neutral gum, and the Olympus upright microscope Image pro plus6.0 software was taken...
Embodiment 2
[0020] Example 2: Effect of Ginsenoside F1 on Inflammatory Response in ApoE- / - Mice
[0021] The bioluminescent MPO inflammatory probe was used as an indicator, and the probe was injected intraperitoneally for 15 minutes to detect the generation of inflammatory mediators in the mouse body using the Jinguozhen spectrum small animal live imaging. The excitation light wavelength was 750λm, and the total luminous intensity was measured. The results are shown in figure 2 .
[0022] The luminescence intensity of the ApoE- / - model group was 2.9 times that of the C57 blank group, while the F1 administration group could reduce the luminescence intensity to 1.5 times that of the blank group.
Embodiment 3
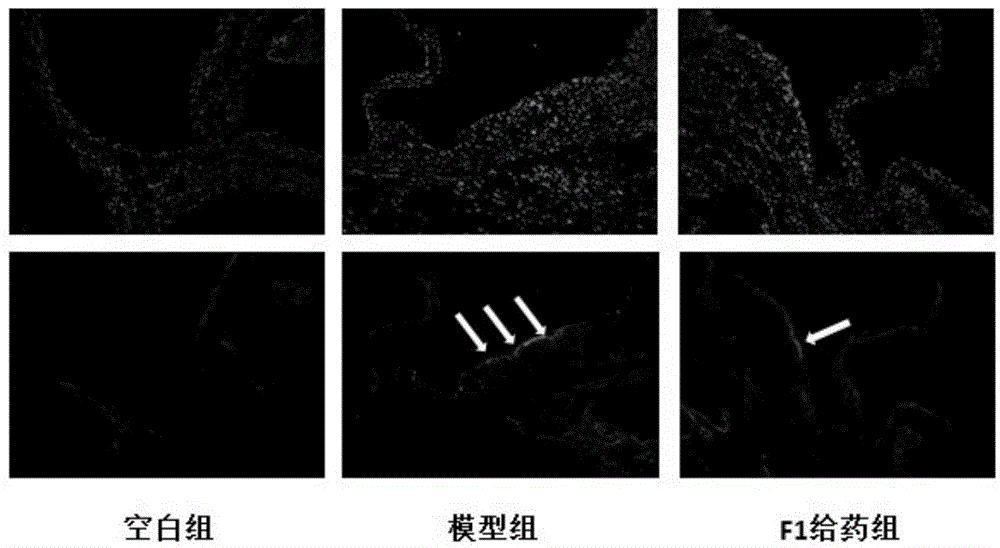
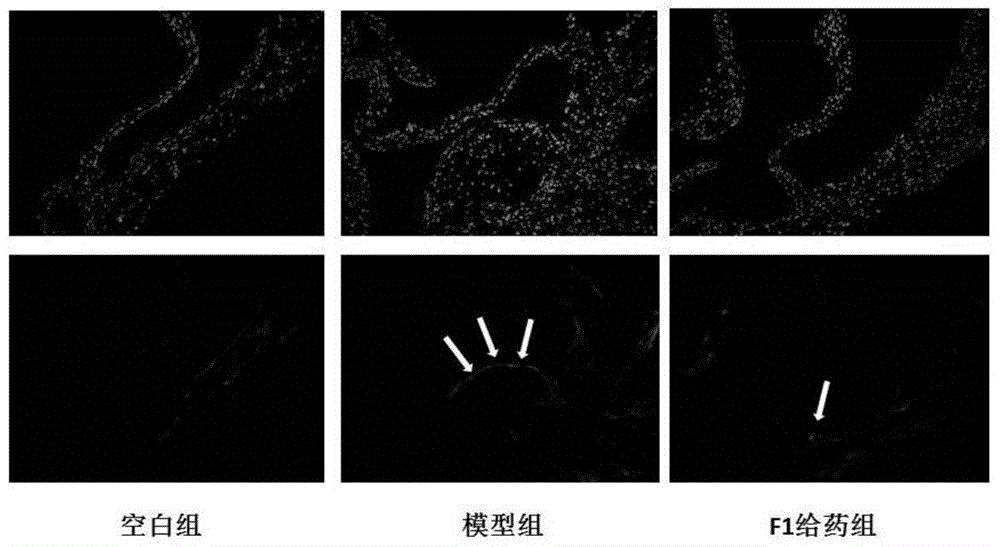
[0023] Example 3: Effect of ginsenoside F1 on LOX-1 and TLR-4 receptors in the aortic arch intima of ApoE- / - mice
[0024] Frozen sections of ApoE- / - mouse aortic arch were placed at room temperature for 30 minutes, washed with water, equilibrated with PBS for 10 minutes, blocked with goat serum for 1 hour, incubated with primary antibody for 1 hour at room temperature, washed 3 times with PBS for 5 minutes each, and incubated with secondary antibody with FITC at room temperature in the dark. After 1 hour, wash with PBS for 3 times, each time for 5 minutes, seal the slides with DAPI-containing anti-fluorescence quenching mounting medium, observe quickly, and take photos with Olympus fluorescence inverted microscope at 100 times magnification. The results are shown in image 3 and 4 .
[0025] LOX-1 and TLR-4 were highly expressed on the intima of the aortic arch in the model group, and the expression of these two receptors could be significantly reduced in the F1 group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com