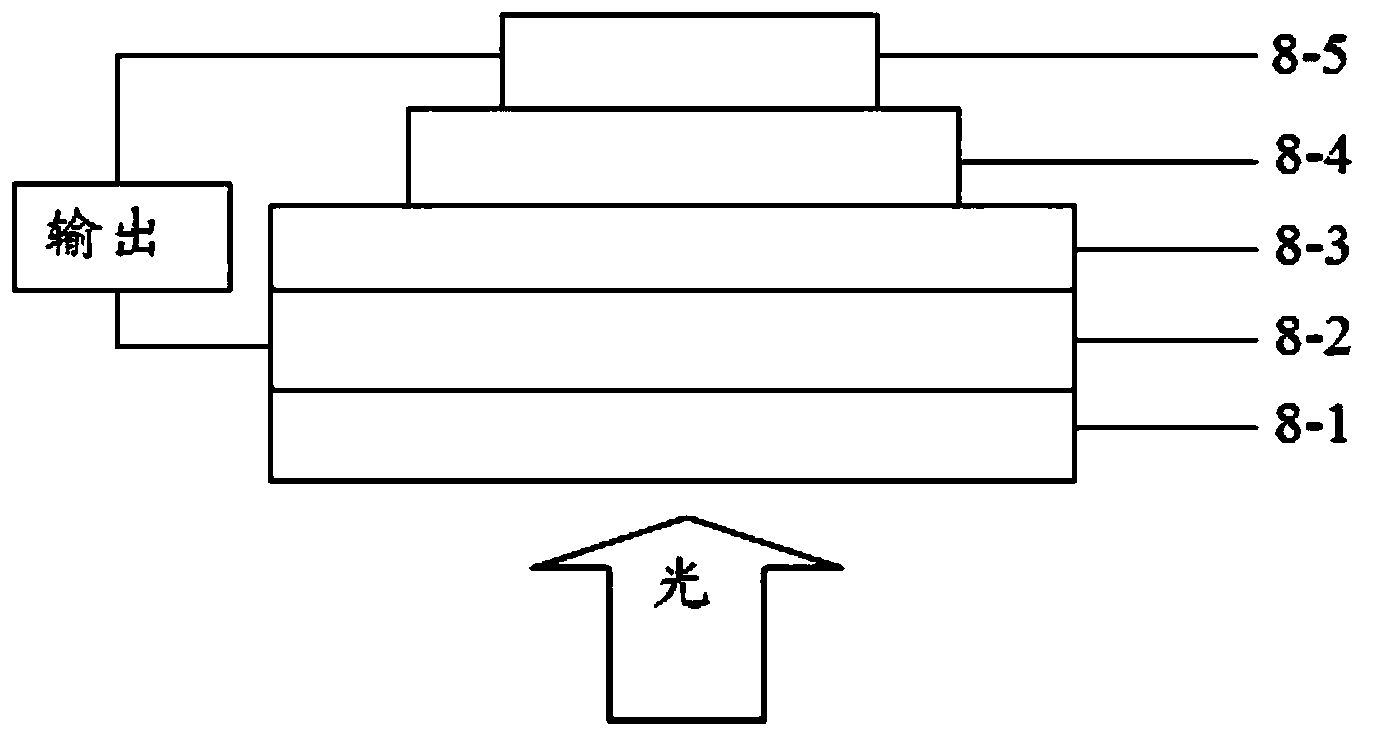
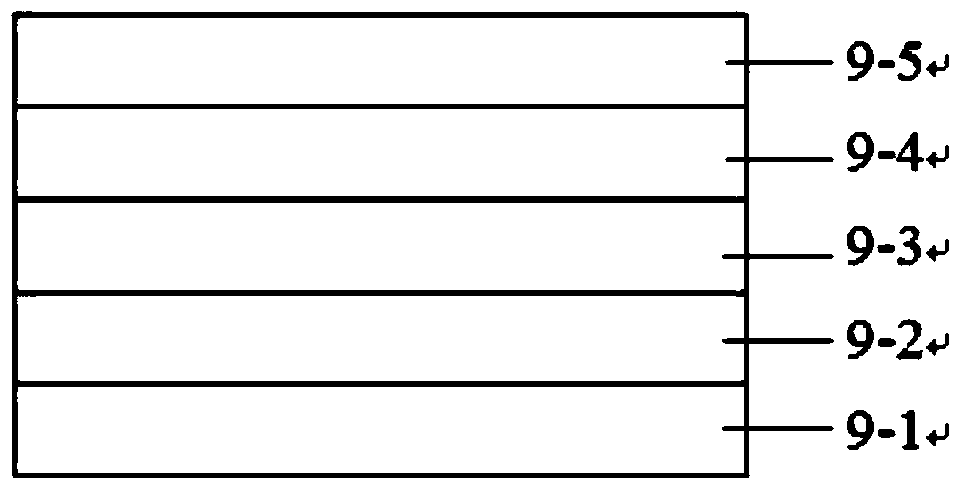
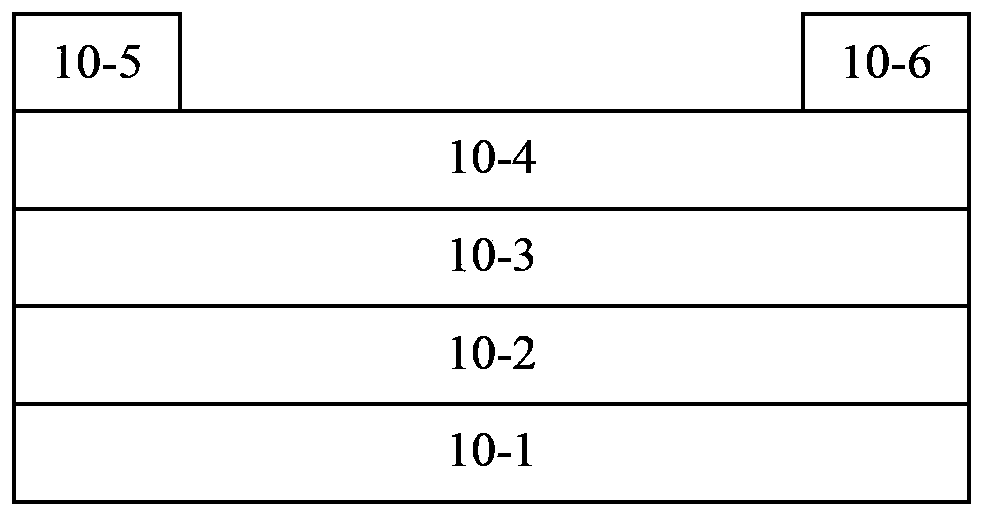
Polymer containing thienothiophene-cyclopentadithiophene, preparation method and application thereof
A technology of dithiophene and polymers, which is applied in the field of polymers containing thienothiophene-cyclopentadithiophene and its preparation, which can solve the problems of poor matching between absorption spectrum and solar spectrum, low photoelectric conversion efficiency, and energy conversion Low efficiency and other issues, to achieve excellent solar light matching and carrier transfer performance, improve thermal stability and environmental stability, and high energy conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] A polymer containing thienothiophene-cyclopentadithiophene, specifically polymer P1 having the following general formula, wherein n=70:
[0059]
[0060] The preparation method comprises the following steps:
[0061] 1. 2,6-bis(3-hexyl-5-trimethyltin-2-thiophene)-4,4-[3',3"-bis(dodecyloxy)]biphenylcyclopentacene The preparation method of [2,1-b;3,4-b']dithiophene (B1) comprises the following steps:
[0062] (1), 2,6-dibromo-4,4-[3',3"-bis(dodecyloxy)]biphenylcyclopenta[2,1-b;3,4-b' ] The preparation method of dithiophene (d1) comprises the following steps:
[0063] Provides compound a1, which is 2-bromo-5,3'-bis(dodecyloxy)biphenyl, provides compound b, which is 2,6-dibromocyclopenta[2,1-b;3,4- b'] dithiophen-4-one;
[0064] Under nitrogen protection, add 3.0g (5mmol) of compound a1 into 60mL of anhydrous tetrahydrofuran in a reaction flask to obtain a solution, cool to -78°C, and slowly add 3mL of n-butyllithium in n-hexane (2.5mol / L, 7.5mmol), the addition was...
Embodiment 2
[0080] A polymer containing thienothiophene-cyclopentadithiophene, specifically a polymer P2 having the following general formula, wherein n=40:
[0081]
[0082] The preparation method comprises the following steps:
[0083] 1. 2,6-bis(2-trimethyltin-3-dodecyl-5-thiophene)-4,4-[3',3"-bis(decyloxy)]biphenylcyclopentadiene The preparation method of [2,1-b; 3,4-b'] dithiophene (B2) comprises the following steps:
[0084] (1), 2,6-dibromo-4,4-[3',3"-bis(decyloxy)]biphenylcyclopenta[2,1-b;3,4-b']di The preparation method of thiophene (d2) comprises the following steps:
[0085] Provides compound a2, which is 2-bromo-5,3'-bis(decyloxy)biphenyl, provides compound b, which is 2,6-dibromocyclopenta[2,1-b;3,4-b' ]dithiophen-4-one;
[0086] Under nitrogen protection, 2.73g (5mmol) of compound a2 was added to 60mL of anhydrous tetrahydrofuran in a reaction flask to obtain a solution, cooled to -78°C, and 2.4mL of n-butyllithium in n-hexane (2.5mol / L , 6mmol), after the addition w...
Embodiment 3
[0102] A polymer containing thienothiophene-cyclopentadithiophene, specifically polymer P3 having the following general formula, wherein n=10:
[0103]
[0104] The preparation method comprises the following steps:
[0105] 1. 2,6-bis(5-trimethyltin-2-thiophene)-4,4-biphenylcyclopenta[2,1-b;3,4-b']dithiophene (B3) The preparation method comprises the following steps:
[0106] (1), the preparation method of 2,6-dibromo-4,4-biphenylcyclopenta[2,1-b; 3,4-b']dithiophene (d3) comprises the following steps:
[0107] Provide compound a3, namely 2-bromo-biphenyl, provide compound b, namely 2,6-dibromocyclopenta[2,1-b;3,4-b']dithiophen-4-one;
[0108] Under nitrogen protection, add 1.17g (5mmol) of compound a3 into 60mL of anhydrous tetrahydrofuran in a reaction flask to obtain a solution, cool to -78°C, and slowly add 2.6mL of n-butyllithium in n-hexane (2.5mol / L , 6.5mmol), the addition was completed, and after the reaction was stirred at -78°C for 1h, 2.1g (6mmol) of compound ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Short circuit current density | aaaaa | aaaaa |
| Maximum brightness efficiency | aaaaa | aaaaa |
| Maximum brightness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com