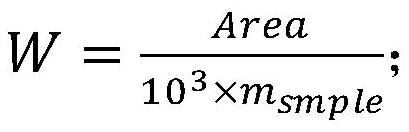Patents
Literature
55 results about "Ciclopentolato" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
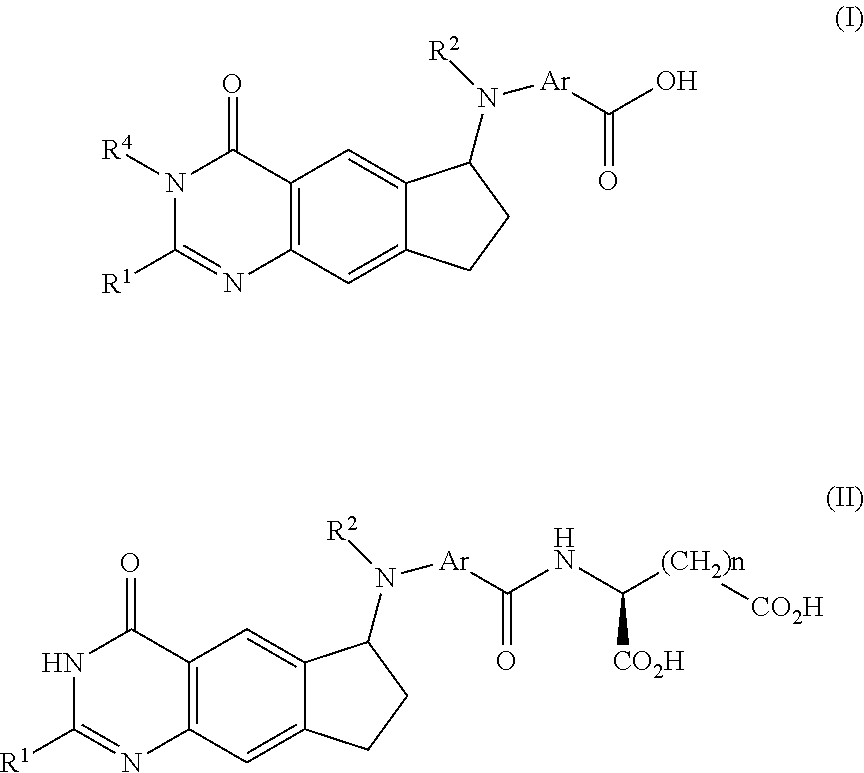
Octahydrocyclopentapyrroles, their preparation and use
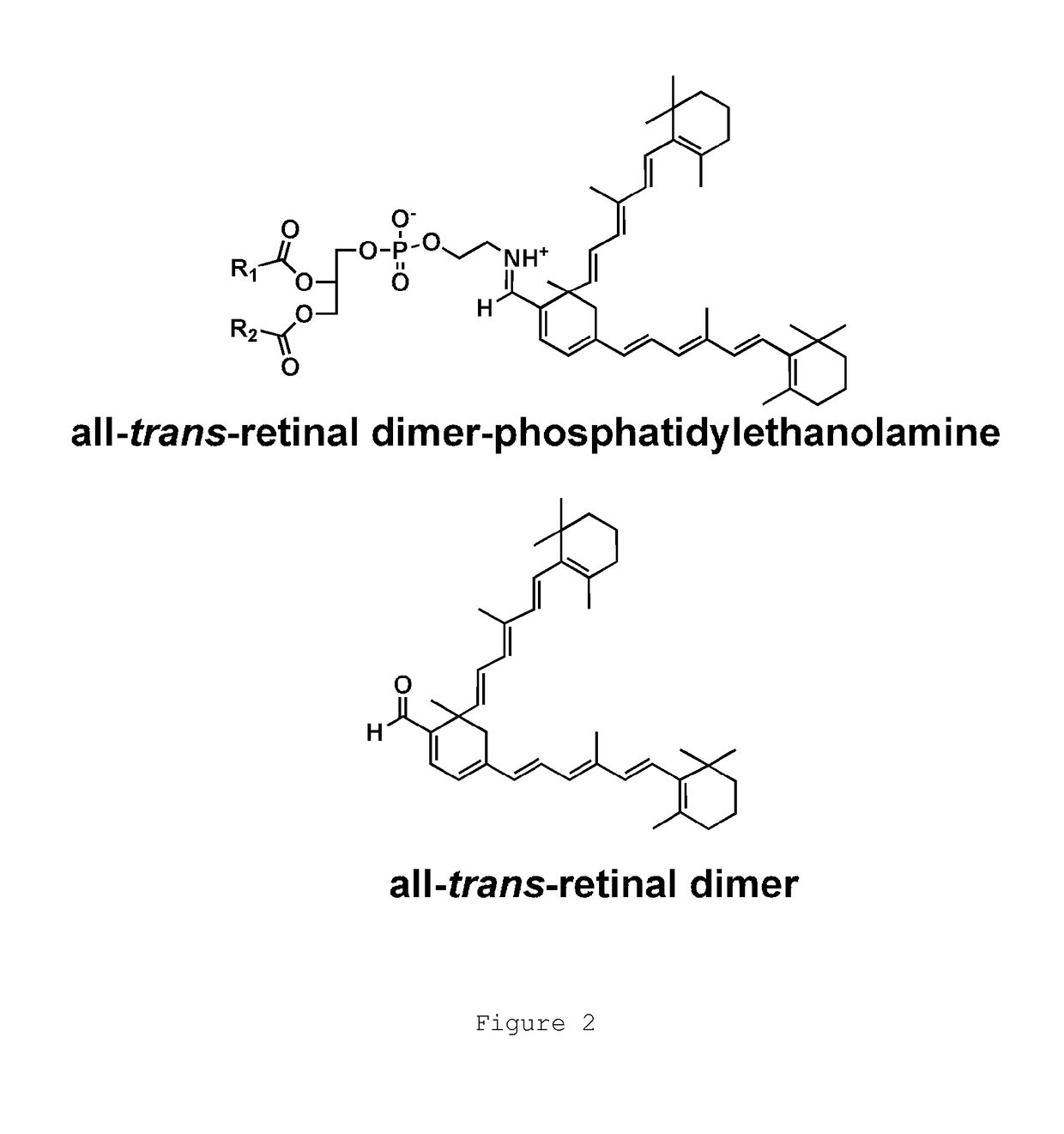
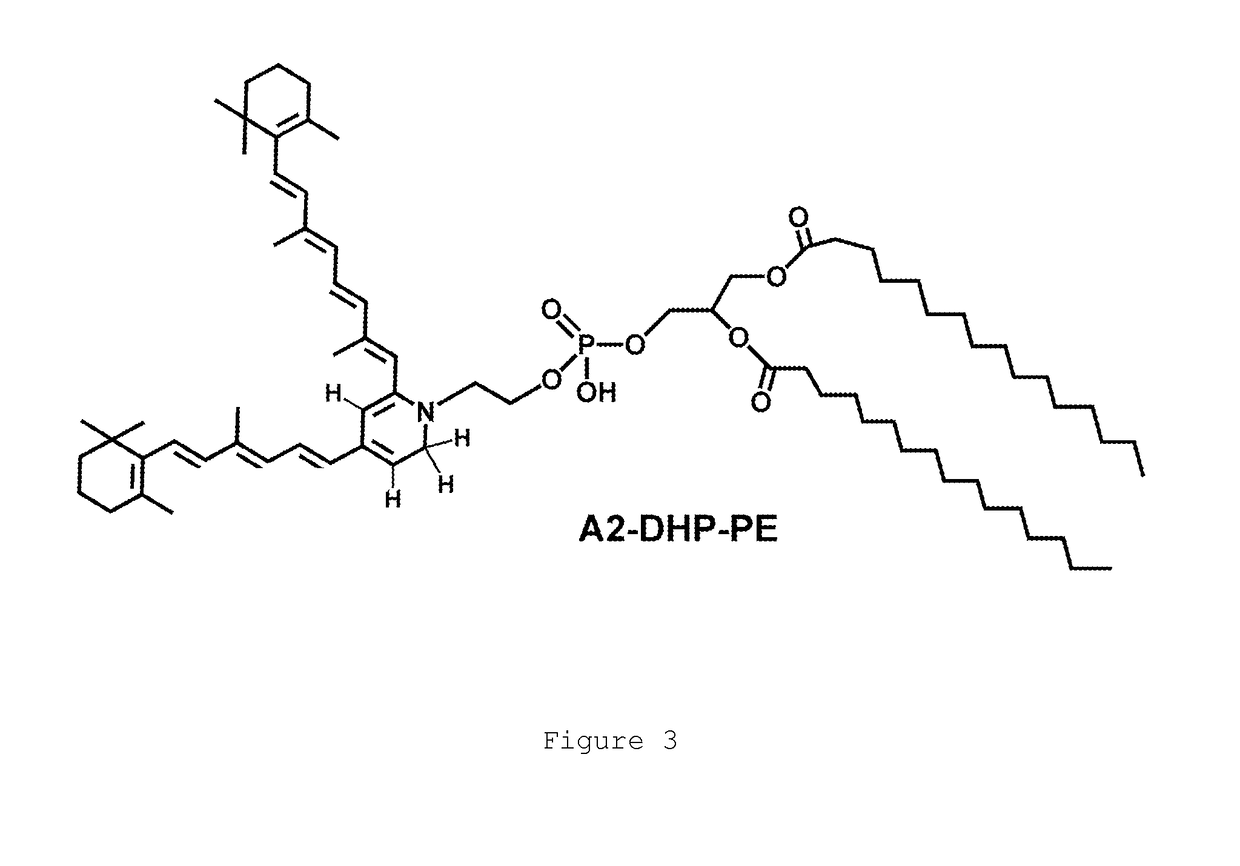
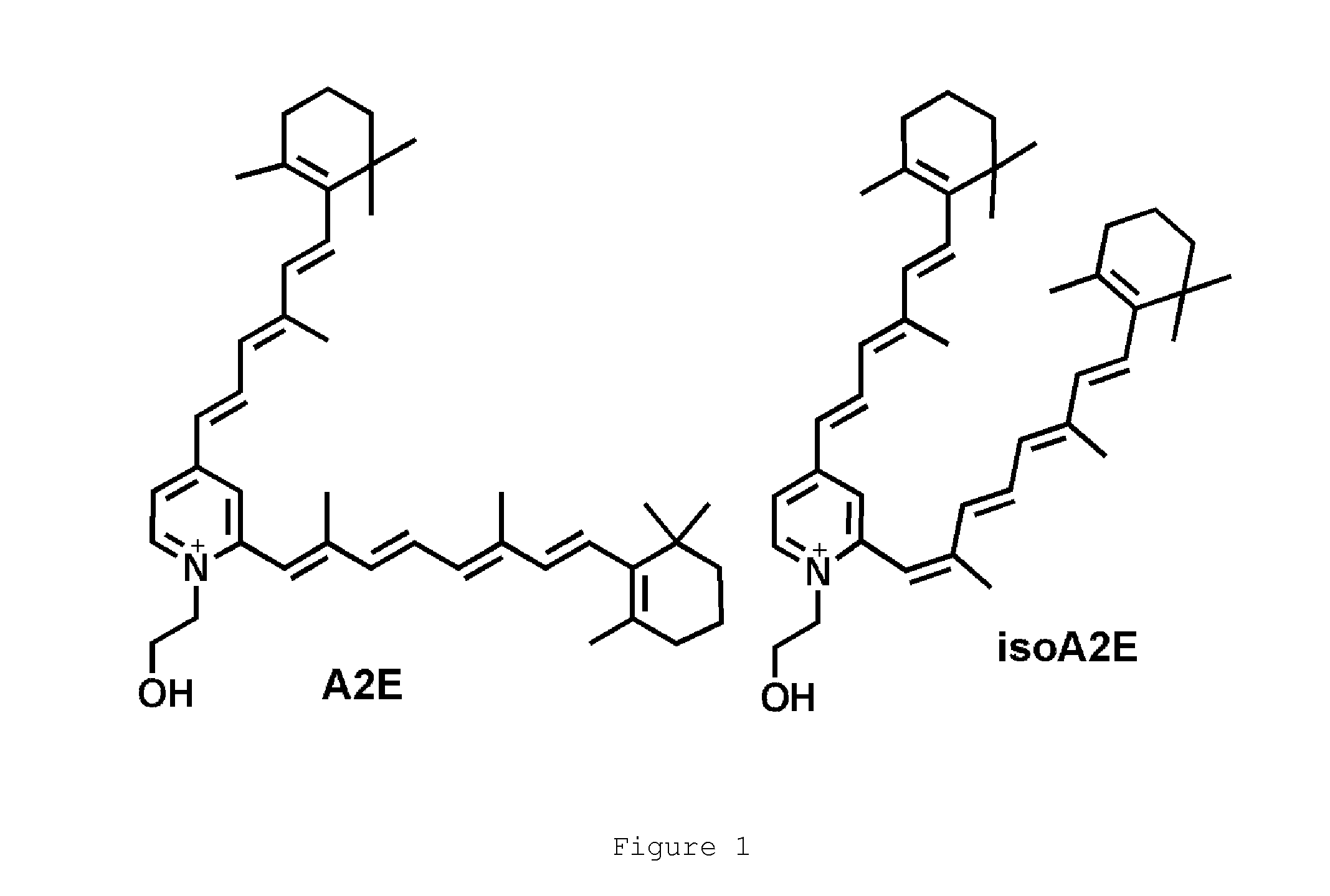
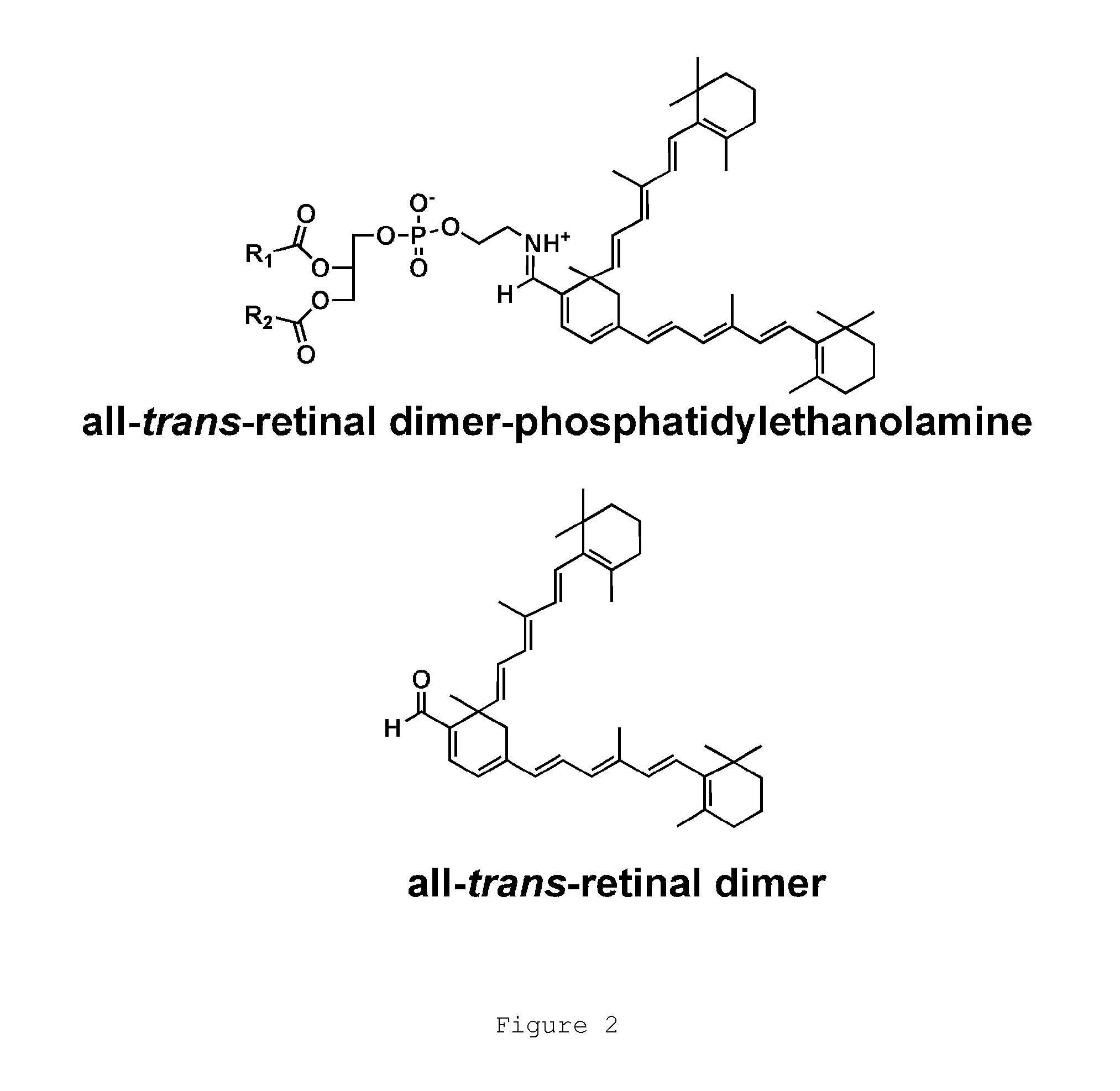
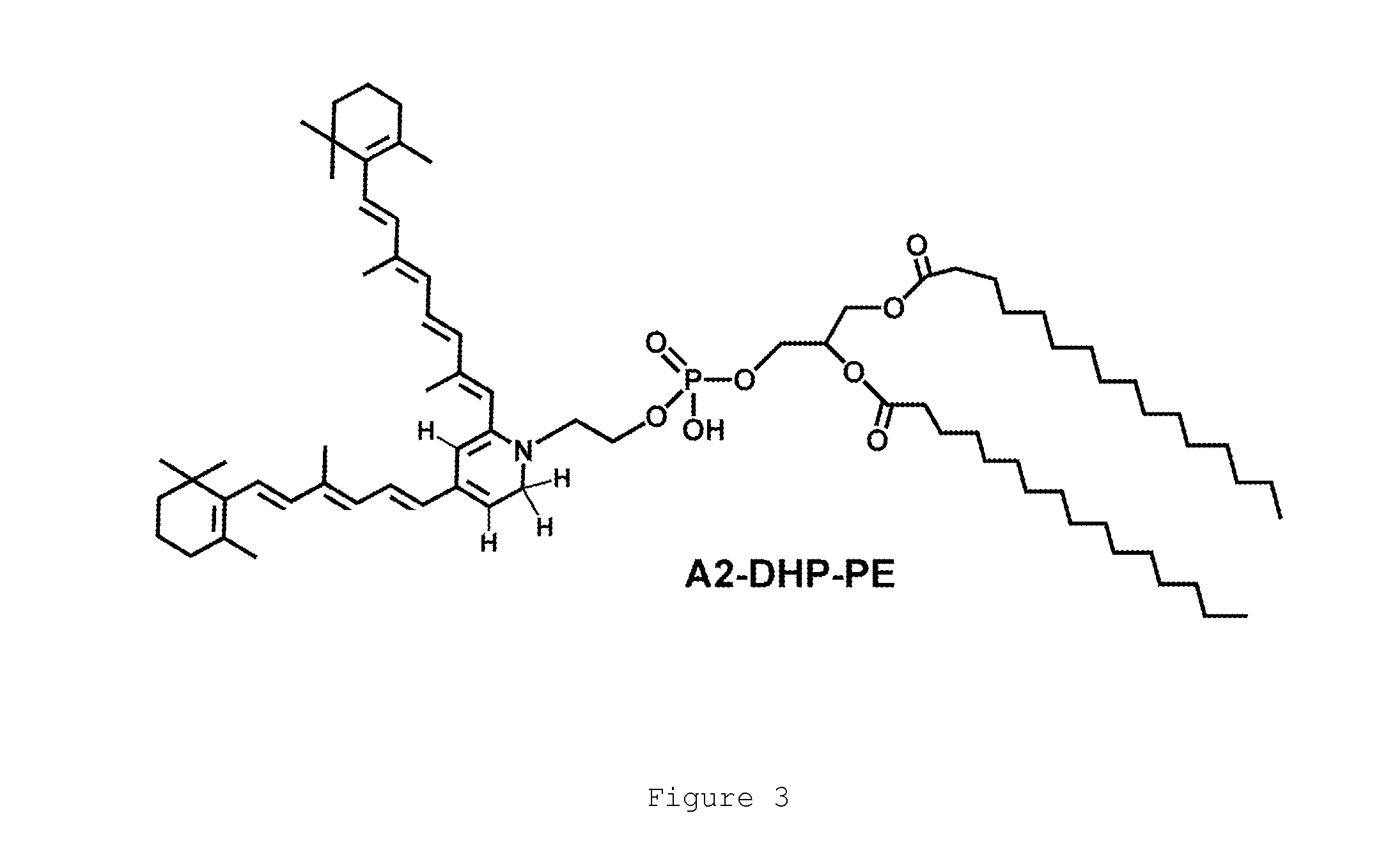
The present invention provides Octahydrocyclopentapyrrole compounds having the structure: (structurally represented) wherein psi is absent or present, and when present is a bond; R1, R2, R3, R4, and R5 are each independently H, halogen, CF, or C1-C4 alkyl; R6 is absent or present, and when present is H, OH, or halogen; A is absent or present, and when present is C(O) or C(O)NH; B is substituted or unsubstituted monocycle, bicycle, heteromonocycle, heterobicycle, benzyl, CO2H or (C1-C4 alkyl)-CO2H, wherein when B is CO2H, then A is present and is C(O); and when psi is present, then R6 is absent and when psi is absent, then R6 is present, or a pharmaceutically acceptable salt thereof, for treatment of diseases characterized by excessive lipofuscin accumulation in the retina.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Process for the preparation of 1-[cyano(aryl)methyl] cyclohexanol
InactiveUS20020120164A1Easy to optimizeReduce usageCarboxylic acid nitrile preparationOrganic compound preparationCyclohexanoneCarbanion
A process for the preparation of 1-[(cyano) aryl methyl] cyclohexanol of the general formula 1 (1a-d) by reacting cyclohexanone with the carbanions of an aryl acetonitrile of the general formula 3 (3a-d), a) R1=H, R2=H b) R1=OMe R2=H c) R1=OMe R2=OMe d) R1=OMe R2=cyclopentyloxy using a base, isolating the compound of formula I and purifying the compound of formula 1a-d by crystallisation is disclosed.
Owner:COUNCIL OF SCI & IND RES
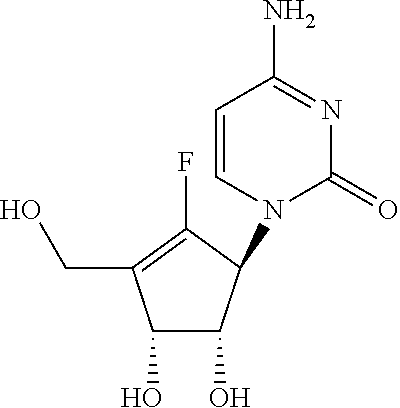
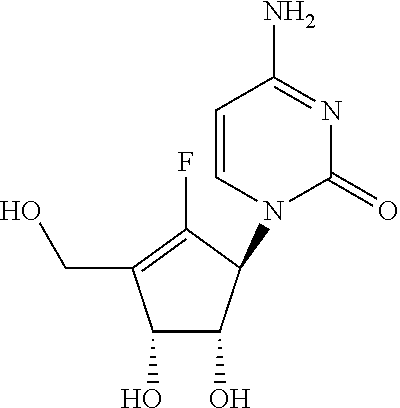
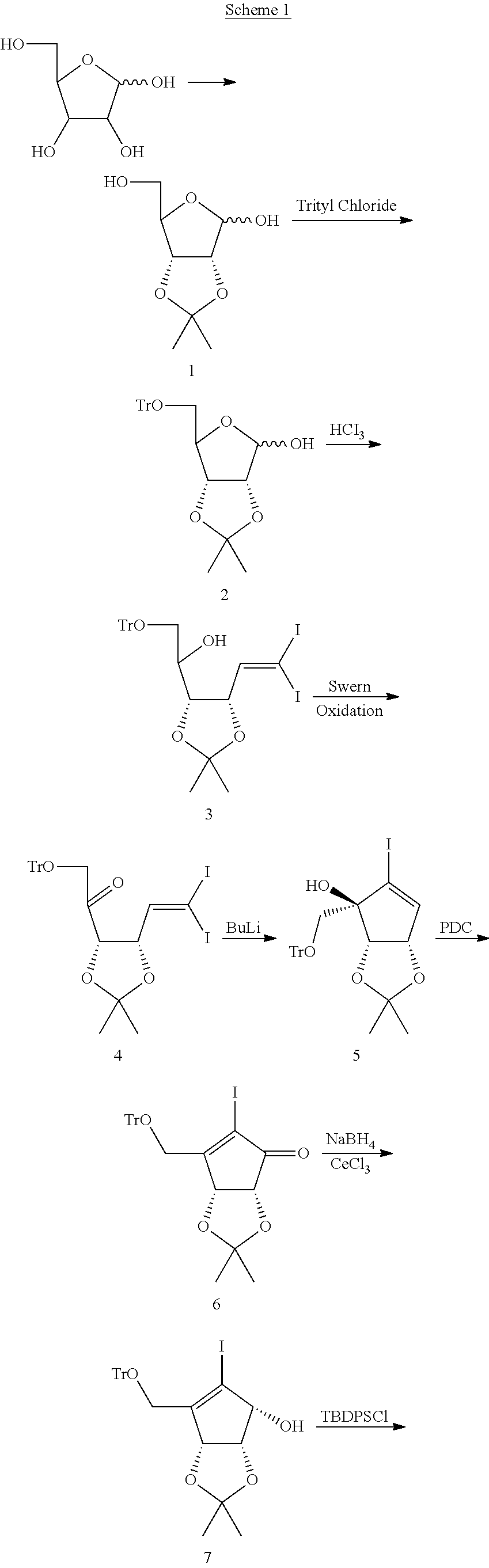
Process for the preparation of 4-amino-1-((1S,4R,5S)-2-fluoro-4,5-dihydroxy-3-hydroxymethyl-cyclopent-2-enyl)-1H-pyrimidin-2-one
ActiveUS9150520B2Group 4/14 element organic compoundsOrganic chemistry methodsCiclopentolatoHydroxy group
Processes for the preparation of 4-amino-1-((1S,4R,5S)-2-fluoro-4,5-dihydroxy-3-hydroxymethyl-cyclopent-2-enyl)-1H-pyrimidin-2-one (13, RX-3117) and its intermediates are described.
Owner:OCUPHIRE PHARM INC
Polymer containing thienothiophene-cyclopentadithiophene, preparation method and application thereof
ActiveCN103848966AImprove rigidityImproved thermal and environmental stabilityOrganic chemistrySolid-state devicesOrganic electroluminescenceOptoelectronic materials
The present invention provides a polymer containing thienothiophene-cyclopentadithiophene, a preparation method and an application thereof. The polymer is a polymer P having the following general formula, wherein R1 and R2 are the same or different H, C1-C12 alkyl or C1-C12 alkoxy, R3 and R4 are the same or different H or C1-C12 alkyl, R5 is C1-C12 alkyl or C1-C12 alkoxy, R6 is H or F, and n is a natural number of 10-70. According to the present invention, the polymer contains the new cyclopentadithiophene and thienothiophene unit, has the new conjugate plane structure, has characteristics of excellent sunlight matching property, excellent carrier mobility property and simple and controllable preparation method, and has good application prospects in the fields of polymer solar cells, organic electroluminescent devices and other optoelectronic materials. The polymer P is as the follow.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
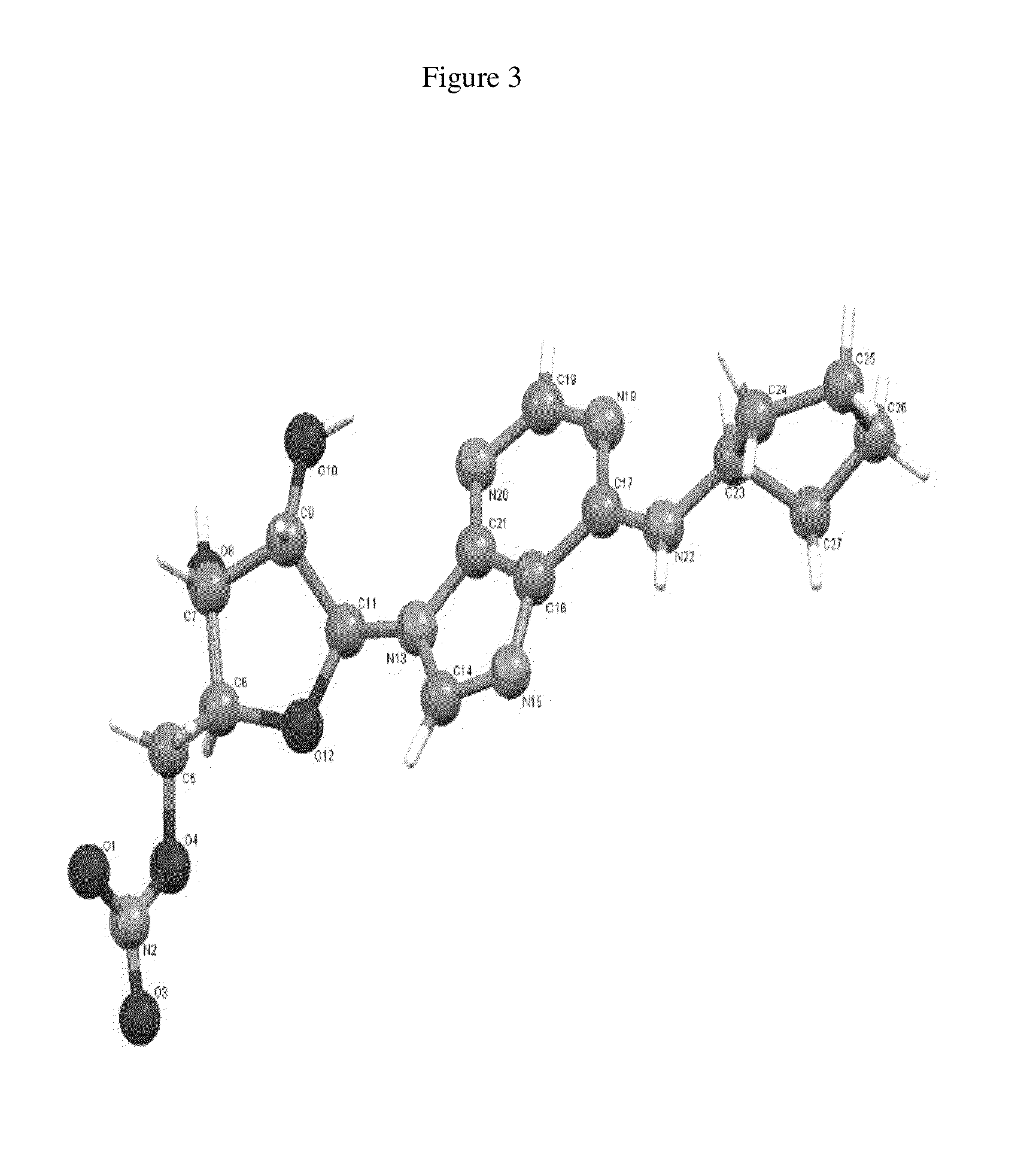
Cyclopenta[b]indole derivatives as sPLA2 inhibitors
InactiveUS7160909B2Potent and selective effectivenessDissolve fastAntibacterial agentsBiocideMedicineCiclopentolato
A novel class of tricyclic compounds of the following formula (I) is disclosed together with the use of such compounds for inhibiting sPLA2 mediated release of fatty acids for treatment of Inflammatory Diseases such as septic shock.
Owner:ELI LILLY & CO
Octahydrocyclopentapyrroles, their preparation and use
The present invention provides Octahydrocyclopentapyrrole compounds having the structure: (structurally represented) wherein psi is absent or present, and when present is a bond; R1, R2, R3, R4, and R5 are each independently H, halogen, CF, or C1-C4 alkyl; R6 is absent or present, and when present is H, OH, or halogen; A is absent or present, and when present is C(O) or C(O)NH; B is substituted or unsubstituted monocycle, bicycle, heteromonocycle, heterobicycle, benzyl, CO2H or (C1-C4 alkyl)-CO2H, wherein when B is CO2H, then A is present and is C(O); and when psi is present, then R6 is absent and when psi is absent, then R6 is present, or a pharmaceutically acceptable salt thereof, for treatment of diseases characterized by excessive lipofuscin accumulation in the retina.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
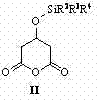
Preparation method of (3R,5R)-3,5-dihydroxy-6-methyl cyan-caproate
InactiveCN102827030AHigh yieldHigh stereoselectivityCarboxylic acid nitrile preparationOrganic compound preparationCyanide compoundPtru catalyst
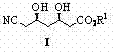
The invention relates to a preparation method of (3R, 5R)-3,5-dihydroxy-6-methyl cyan-caproate (I) and belongs to the technical field of pharmaceutical chemistry. The preparation method concretely comprises the following steps of: firstly, carrying out asymmetric catalysis alcoholysis on 3-siloxy cyclopentane anhydride (II) to prepare (R)-3-siloxy-5-alkoxy-5-oxo-pentanoate (III); secondly, condensing the (R)-3-siloxy-5-alkoxy-5-oxo-pentanoate (III) and methyl cyanoacetate to prepare (R)-2-cyano-3-oxo-5-siloxy diethyl pimelate (IV); thirdly, carrying out decarboxylation on the (R)-2-cyano-3-oxo-5-siloxy diethyl pimelate (IV) to prepare (R)-3-hydroxy-5-oxo-6-benzyl cyanohexanoate (V) by using desilicication protective groups; and fourthly, carrying out asymmetric reduction on the (R)-3-hydroxy-5-oxo-6-benzyl cyanohexanoate to prepare a product of (3R,5R)-3,5-dihydroxy-6-methyl cyan-caproate (I). The preparation method is mild in reaction conditions, simple and convenient to operate, high in stereoselectivity, environment-friendly, and suitable for industrial production; and products have high yield, the used chiral catalyst is small in dosage and can be recovered with fix quantify, raw materials which are easily obtained are low in cost, and particularly hypertoxic cyanides are avoided.
Owner:FUDAN UNIV
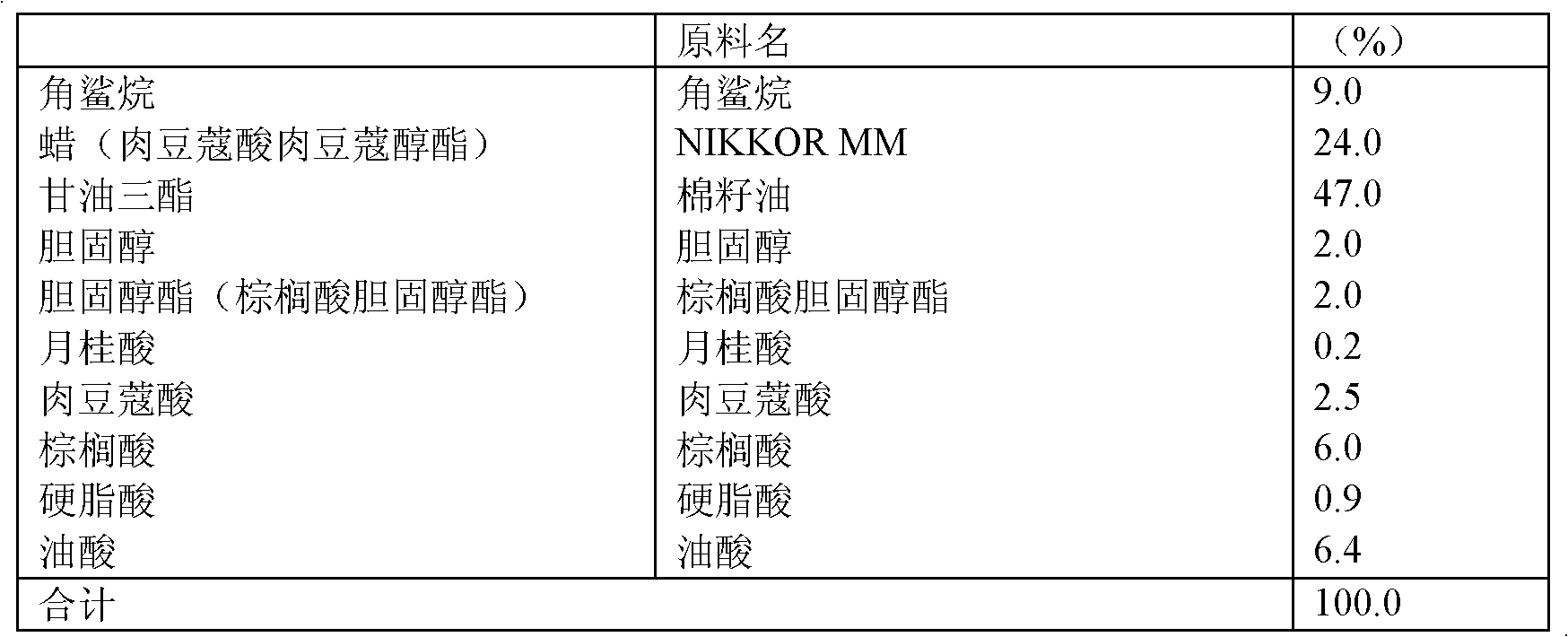
Oil phase dispersion sunblocking concentrate of nanometer titania and preparation method thereof
InactiveCN102228412AHigh solid contentLow viscosityCosmetic preparationsToilet preparationsUltravioletCAPRYLIC TRIGLYCERIDE
The invention discloses an oil phase dispersion sunblocking concentrate of nanometer titania and a preparation method as well as application thereof. The concentrate of the invention comprises the following materials by weight: (a) 35-60% of nanometer titania; (b) 30-62% of organic solvent which is one or more of dimethyl polysiloxane, cyclopentasiloxane, alkyl benzoate, caprylic triglyceride and capric triglyceride; (c) 1-10% of dispersing accessory which is one or more of polyhydroxystearate, polyhydroxystearic acid and polyglyceryl polysiloxane. Characterized by uniformity and stability, small viscosity, high content of effective components, obvious blocking effect against ultraviolet rays and complete permeability to visible light, the concentrate provided in the invention is beneficial for the production and processing of various sunblocking cosmetics.
Owner:蒲科
Polymer containing thienothiophene, benzothiadiazole and cyclopentadithiophene, preparation method and application thereof
ActiveCN103848967ALower bandgapImprove rigidityOrganic chemistrySolid-state devicesPolymer scienceTerthiophene
The present invention provides a polymer containing thienothiophene, benzothiadiazole and cyclopentadithiophene, a preparation method and an application thereof. The polymer is a polymer P having the following general formula, wherein R1 and R2 are the same or different H or C1-C12 alkyl, and n is a natural number of 5-60. According to the present invention, the three units such as thienothiophene, benzothiadiazole and cyclopentadithiophene in the polymer are combined to form the repeated unit having the acceptor-donor-acceptor-donor (A-D-A-D) form so as to enhance the charge transporting in the polymer molecule, reduce the band gap of the polymer, and expand the light absorption range of the polymer material, such that the polymer has characteristics of excellent sunlight matching property, excellent carrier mobility property and simple and controllable preparation method, and has good application prospects in the fields of polymer solar cells, organic electroluminescent devices and other optoelectronic materials. The polymer P is as the follow.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Process for production of 3-[5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-oxo-2-substituted-2,3-dihydro-1,2-benzisoxazol-6-yl)methoxy]phenyl]propionate ester and intermediate for the process
InactiveUS20090099369A1Easy to prepareReduce environmental impactPreparation by ester-hydroxy reactionOrganic compound preparationPropionatePropanoic acid
A preparation method using as an intermediate 6-(halomethyl)-1,2-benzisoxazol-3(2H)-one derivative represented by general formulawherein R5 is a methyl group that is substituted with one or more optionally substituted phenyl groups, or an optionally substituted oxygen-containing heterocyclic group; X represents a halogen atom, can be used as a method for safely and easily preparing 3-{5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-hydroxy-1,2-benzisoxazol-6-yl)methoxy]phenyl}propionic acid, which is useful as an antirheumatic agent, with a high yield.
Owner:TOYAMA CHEM CO LTD
Water-in-oil type emulsified cosmetic and skin care method
The invention provides a water-in-oil type emulsified cosmetic which is excellent in stability, isolation effect and skin feeling. The water-in-oil type emulsified cosmetic is prepared by comprising the following components: (A) 0.5-10wt% of a silicone elastomer, (B) 0.2-10wt% of dimethyl polysiloxane with the polymerization degree of more than 350 and less than 1500, (C) 3-28wt% of dimethyl polysiloxane and / or decamethyl cyclopentasiloxane with the polymerization degree of more than 1 and less than 6, (D) 0.5-25wt% of ethanol and (E) water. Furthermore, the invention also provides a skin care method which applies the water-in-oil type emulsified cosmetic provided by the invention to the skin and maintains the skin.
Owner:KAO CORP
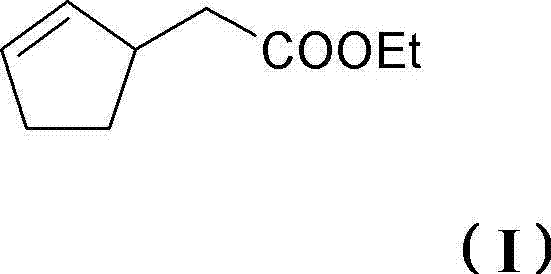
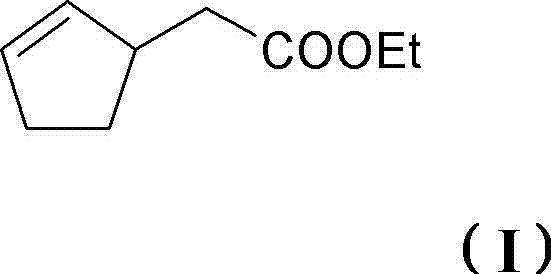
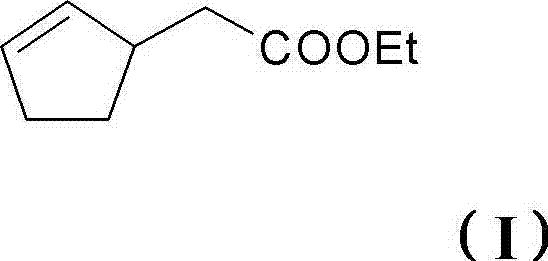
Perfume mixtures containing cyclopent-2-enyl-ethyl acetate
The invention provides a perfume mixture, preferably perfume oil, which comprises: (a) ethyl 2-(cyclopent-2-enyl)acetate (I); and (b) at least one perfume with a flowery olfactory note, comprising alcohols or aldehydes with a molar mass of = 210 g / mole; and / or (c) at least one perfume comprising ketones, ethers or esters with a molar mass of 190-250 g / mole.
Owner:SYMRISE GMBH & CO KG
Amino-substituted rutaecarpin analog, and synthesis method and application thereof in preparation of anti-obesity medicaments
ActiveCN102775413AReduce accumulationReduce obesityOrganic active ingredientsOrganic chemistryRutaecarpineMorpholine
The invention relates to the fields of pharmaceutical chemistry and pharmaceutical therapeutics, and provides an amino-substituted rutaecarpin analog, and a synthesis method and application thereof in the preparation of anti-obesity medicaments. Experiments prove that the amino-substituted rutaecarpin analog provided by the invention can obviously reduce the lipopexia of 3T3-L1 fat cells, and can reduce the high levulose-induced lipopexia of FAO rat liver cells. Cellular level tests show that the compound can reduce lipopexia and has an effect of relieving obesity and alleviating fatty liver. The chemical formula of the amino-substituted rutaecarpin analog is shown in the specification. In the chemical formula, R0 is -NH(CH2)nR2, n is 1, 2, 3, 4 or 5, and R2 is -N(CH3)2, -N(CH2CH3)2, cyclopentylamino or cyclohexylamino; or R0 is morpholinyl, piperazinyl or methylpiperazinyl.
Owner:SUN YAT SEN UNIV
Compound reserpine orally disintegrating tablet for treating hypertension and preparation method of compound reserpine orally disintegrating tablet
InactiveCN105213425AEasy to takeAvoid side effectsOrganic active ingredientsPill deliveryVitamin b6Orally disintegrating tablet
The invention belongs to the field of pharmacy, and particularly relates to a compound reserpine orally disintegrating tablet for treating hypertension and a preparation method of the compound reserpine orally disintegrating tablet. The compound reserpine orally disintegrating tablet is prepared from the following raw and auxiliary materials in parts by weight: 0.03 part of reserpine, 1 part of hydralazine hydrochloride, 0.025 part of cyclopenthiazide, 1.5 parts of hydrochlorothiazide, 2 parts of promethazine hydrochloride, 30 parts of potassium chloride, 5 parts of rutin, 2.5 parts of phosphate chloroquine, 1 part of vitamin B1, 1 part of vitamin B6, 2-10 parts of a stabilizer fumaric acid and other pharmaceutical adjuvants. An experiment result shows that the orally disintegrating tablet is short in onset time, long in medicine duration time, and convenient for treatment of a patient for a long period of time; the medication safety is improved; meanwhile, the prescription is high in process stability, and is free of effects of environmental temperature and humidity; and the difference between batches of the preparations is significantly reduced; and the stability of a sample is improved.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
Application of 3-(benzoyl phenyl) propionic acid derivative in treatment of connexin CX31 mediated skin diseases
InactiveCN104546835ARelieve clinical symptomsReach treatmentOrganic active ingredientsDermatological disorderDiseasePropanoic acid
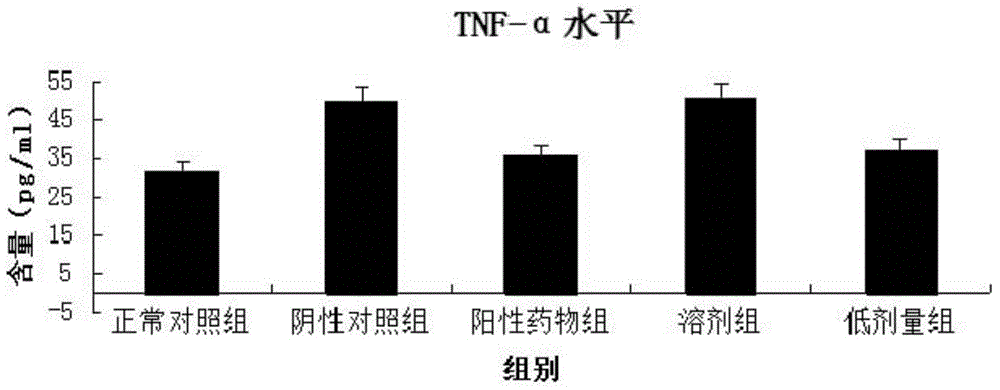
The invention provides an application of a 3-[[2-[3-oxo-(1, 2-benzisoxazole-6-yl) methoxyl]-5-[(2-hydroxyl-4-cyclopentoxyl benzoyl)] phenyl] propionic acid (compound I) in preparation for treatment of connexin CX31 mediated skin disease. The connexin CX31 mediated skin disease comprises erythrokeratodermia variabilis or psoriasis. The pharmacological experiment proves that a low-dose group and a high-dose group of the compound I can remarkably reduce skin damage degree of a psoriasis animal sample; the content of TNF-alpha in murine serum can be restrained by the low-dose group of the compound I, then inflammatory cell infiltration is restrained, the inflammatory response is relieved, and the compound I has good psoriasis treatment effect and can be used for treating the connexin CX31 mediated skin disease.
Owner:TIANJIN INT JOINT ACADEMY OF BIOTECH & MEDICINE +1
Compound reserpine medicine composition for treating hypertension and preparation method of compound reserpine medicine composition
InactiveCN105193841AImprove stabilityImprove standardsPill deliveryPharmaceutical non-active ingredientsVitamin b6Efficacy
The invention belongs to the field of pharmacy, and particularly relates to a compound reserpine medicine composition for treating hypertension and a preparation method of the compound reserpine medicine composition. The compound reserpine medicine composition comprises raw materials in parts by weight as follows: 0.03 parts of reserpine, 1 part of hydralazine hydrochloride, 0.025 parts of cyclopenthiazide, 1.5 parts of hydrochlorothiazide, 2 parts of promethazine hydrochloride, 30 parts of potassium chloride, 5 parts of rutin, 2.5 parts of chloroquine phosphate, 1 part of vitamin B1, 1 part of vitamin B6, 2-10 parts of a stabilizer fumaric acid and other pharmaceutical adjuvants. Experiment results show that the medicine composition takes effect quickly, has long-lasting medicine efficacy, facilitates long-term treatment of a patient and is safe to take; besides, the formula has a high technological stability and is not influenced by environmental temperature and humidity, differences between different bathes of preparations are remarkably reduced, and the stability of samples is improved.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
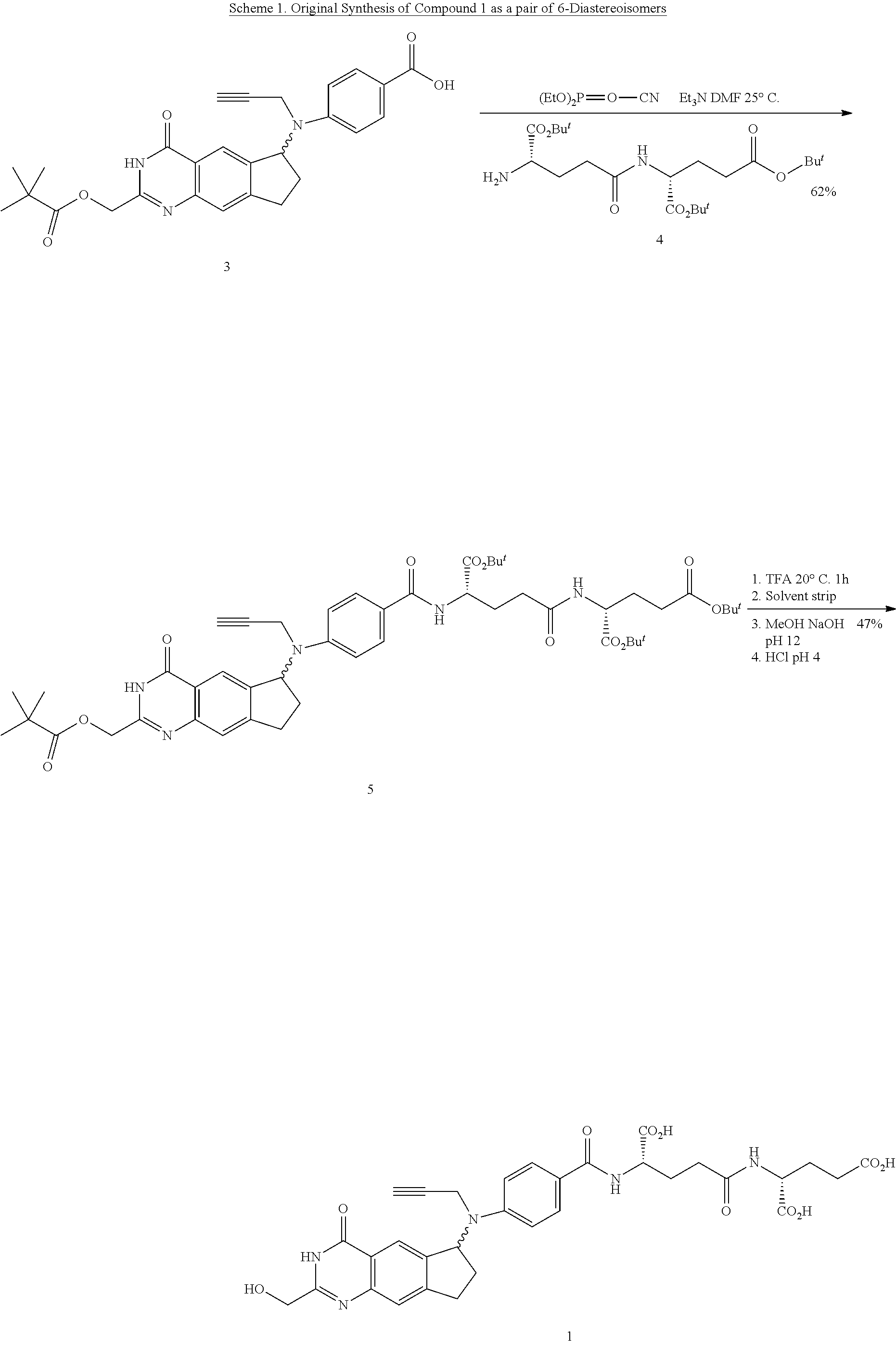
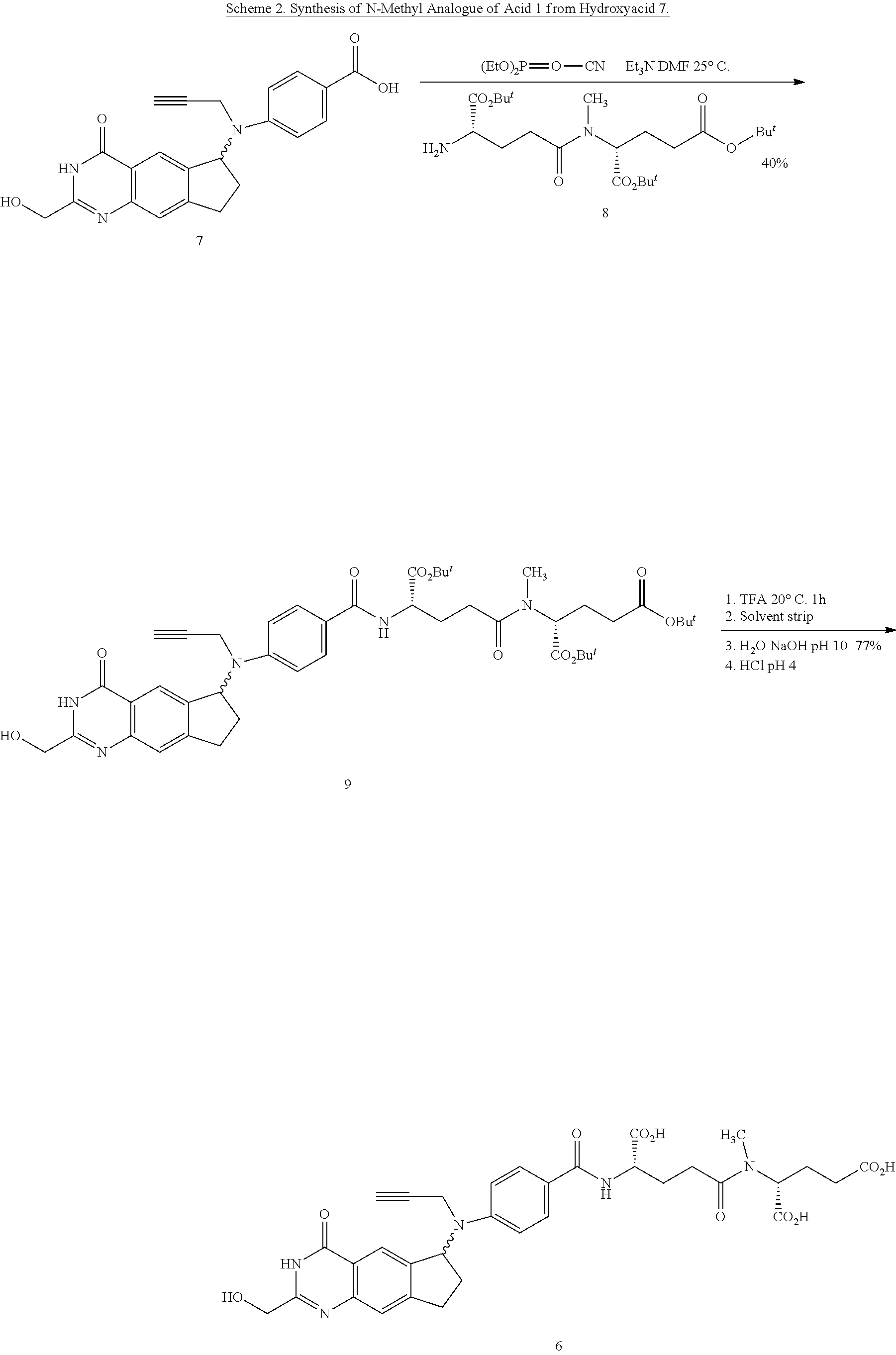
Efficient peptide couplings and their use in the synthesis and isolation of a cyclopenta (g) quinazoline trisodium salt
ActiveUS20130345423A1Long-term useSilicon organic compoundsCarbamic acid derivatives preparationCiclopentolatoCombinatorial chemistry
A new method for the synthesis of L-Glutamyl-γ-D-Glutamic acid and its use in the synthesis of (2R)-((4S)-carboxy-4-(4,N-(((6S)-2-(hydroxymethyl)-4-oxo-3,4,7,8-tetrahydro-3H-cyclopenta[g]quinazolin-6-yl)-N-(prop-2-ynyl)amino)benzamido)butanamido)pentanedioic acid, 1 are provided. Also provided is an efficient method for the isolation and purification of the trisodium salt of the abovementioned acid, 2, in a form suitable for long term storage and use in a parenteral dosing form.
Owner:BTG INT LTD
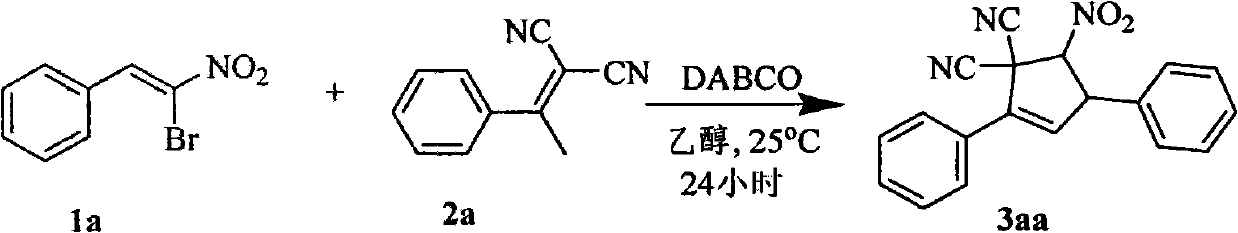
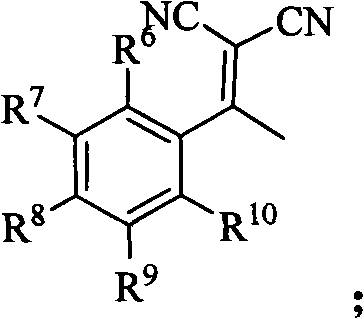
Synthetic method of 5-nitryl-2,4-diphenyl cyclopentyl-2-ene-1,1-dinitrile and derivative thereof
InactiveCN101851177AFlexible response timeHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationBromineStructural formula
The invention discloses a synthetic method of 5-nitryl-2,4-diphenyl cyclopentyl-2-ene-1,1-dinitrile and a derivative thereof, which comprises the following steps of: in the presence of a catalyst, adding substituted or non-substituted 1-bromine-1-nitryl-2-styrene and substituted or non-substituted 1,1-dinitrile-2-methyl-2-styrene into a reaction solvent for reaction, and post processing a reaction product to obtain the 5-nitryl-2,4-diphenyl cyclopentyl-2-ene-1,1-dinitrile and the derivative thereof. The method has the advantages of flexible reaction time, higher yield, low price and easy obtainment of most solvents, environmental protection, simple operation, wide application range and the like and is suitable for industrialized production. The invention also discloses the 5-nitryl-2,4-diphenyl cyclopentyl-2-ene-1,1-dinitrile and the derivative thereof, which have the following structural formulae; and the compound has better antibacterial and insecticidal actions.
Owner:ZHEJIANG NORMAL UNIVERSITY
Anhydrous polymorphs of [(2r,3s,4r,5r)-5-(6-(cyclopentylamino)-9h-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)} methyl nitrate and processes of preparation thereof
The present invention provides novel anhydrous polymorph forms of 2R,3S,4R,5R)-5-(6-(cyclopentylamino)-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl nitrate (Compound A). The present invention also provides processes for preparation of the anhydrous polymorphic forms of compound A.
Owner:INOTECK PHARMA CORP
Preparation method of tetrahydro-cyclopentyl[2,1-b;3,4-b'] bi-thiophene ketone
The invention discloses a preparation method of tetrahydro-cyclopentyl[2,1-b;3,4-b'] bi-thiophene ketone, aiming to solve the problem of lower reaction yield. The invention comprises the following steps of: (1) carrying out a coupling reaction on 2-bromine-3-methyl thiophene and 3.84g of 2-thiophene boric acid to obtain 3-methyl2,2'-bithiophene; (2) carrying out an oxidation reaction on 3-methylbithiophene and potassium permanganate to obtain 2,2'-bithiophene3-formic acid; and (3) reacting the 3-methylbithiophene with concentrated sulfuric acid (98 percent) to obtain the tetrahydro-cyclopentyl[2,1-b;3,4-b'] bi-thiophene ketone. The invention is mainly used for preparing the tetrahydro-cyclopentyl[2,1-b;3,4-b'] bi-thiophene ketone.
Owner:XIAN MODERN CHEM RES INST
Cyclopenta [d] pyrimidine compound, pharmaceutically acceptable salt, solvate or prodrug thereof and application
ActiveCN111303053AInhibition formationInhibit expressionOrganic active ingredientsOrganic chemistryDiseasePharmaceutical drug
The invention discloses a cyclopenta [d] pyrimidine compound, a pharmaceutically acceptable salt, solvate or prodrug thereof, and an application of the cyclopenta [d] pyrimidine compound, the pharmaceutically acceptable salt, the solvate or the prodrug. The compound has a structural formula which is described in the specification. The cyclopenta [d] pyrimidine compound and the pharmaceutically acceptable salt and solvate thereof disclosed by the invention can be used as an HIF-2alpha inhibitor for preparing medicines for treating and / or preventing HIF-2alpha related diseases or symptoms of mammals.
Owner:OCEAN UNIV OF CHINA
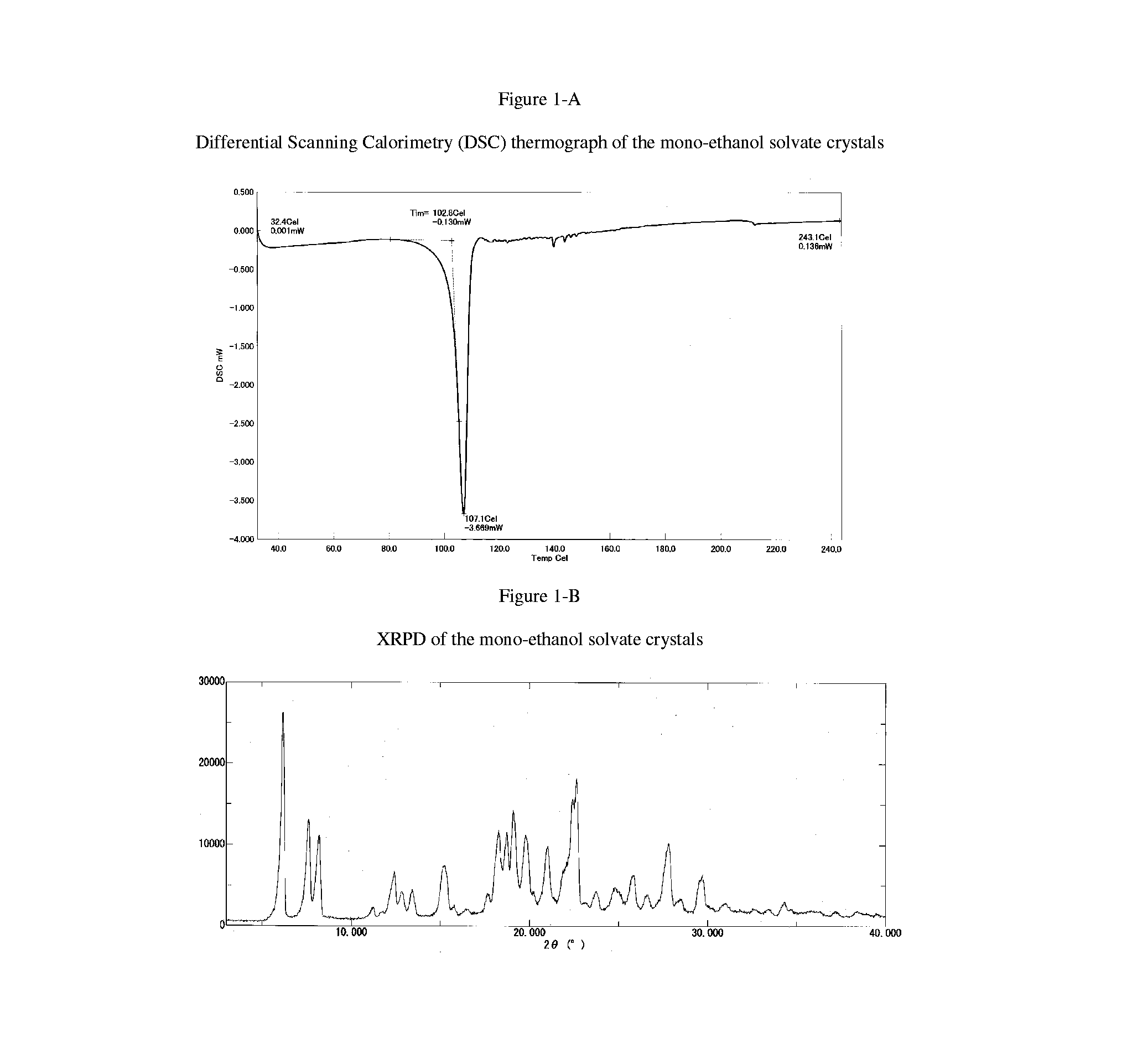
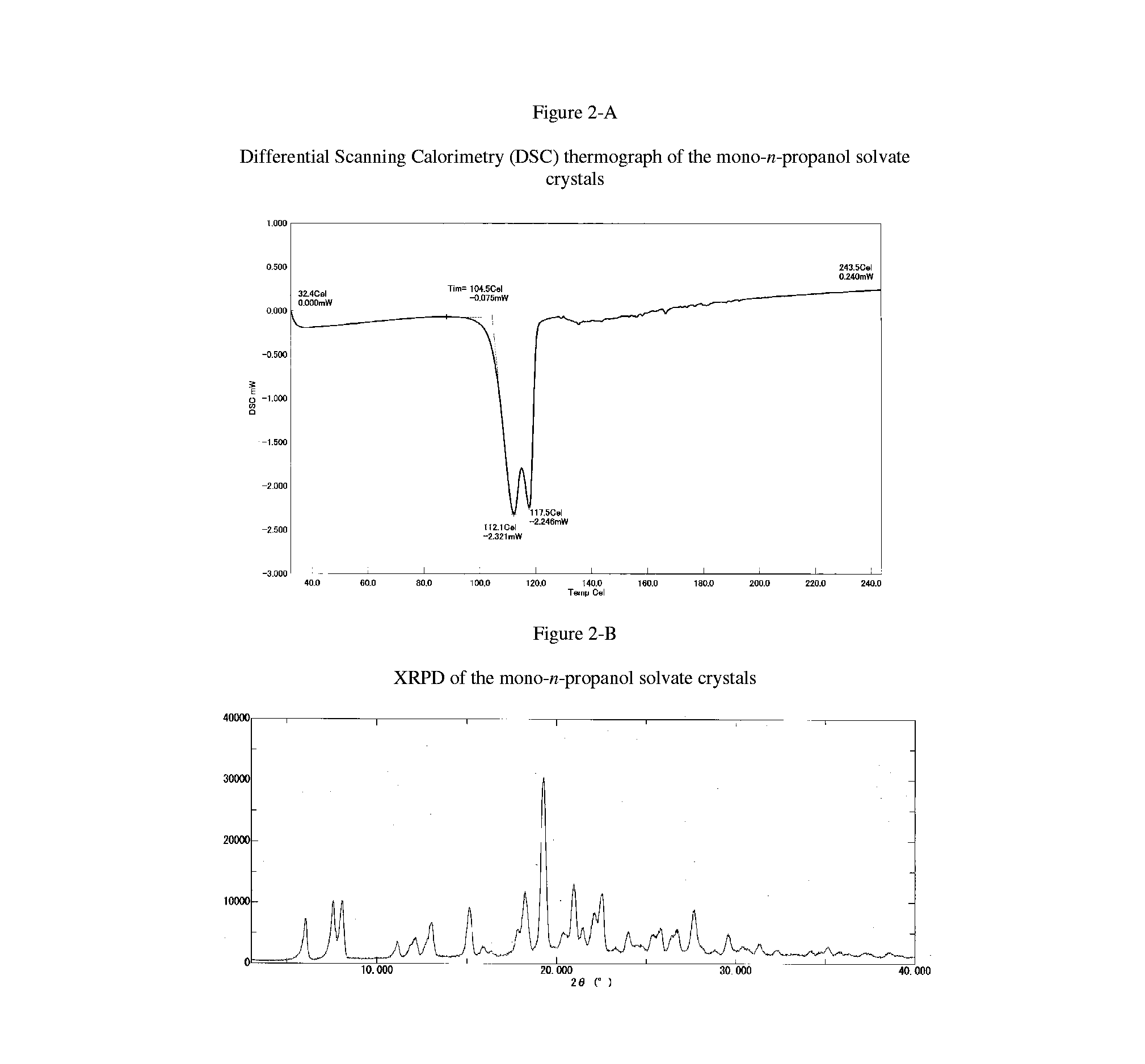
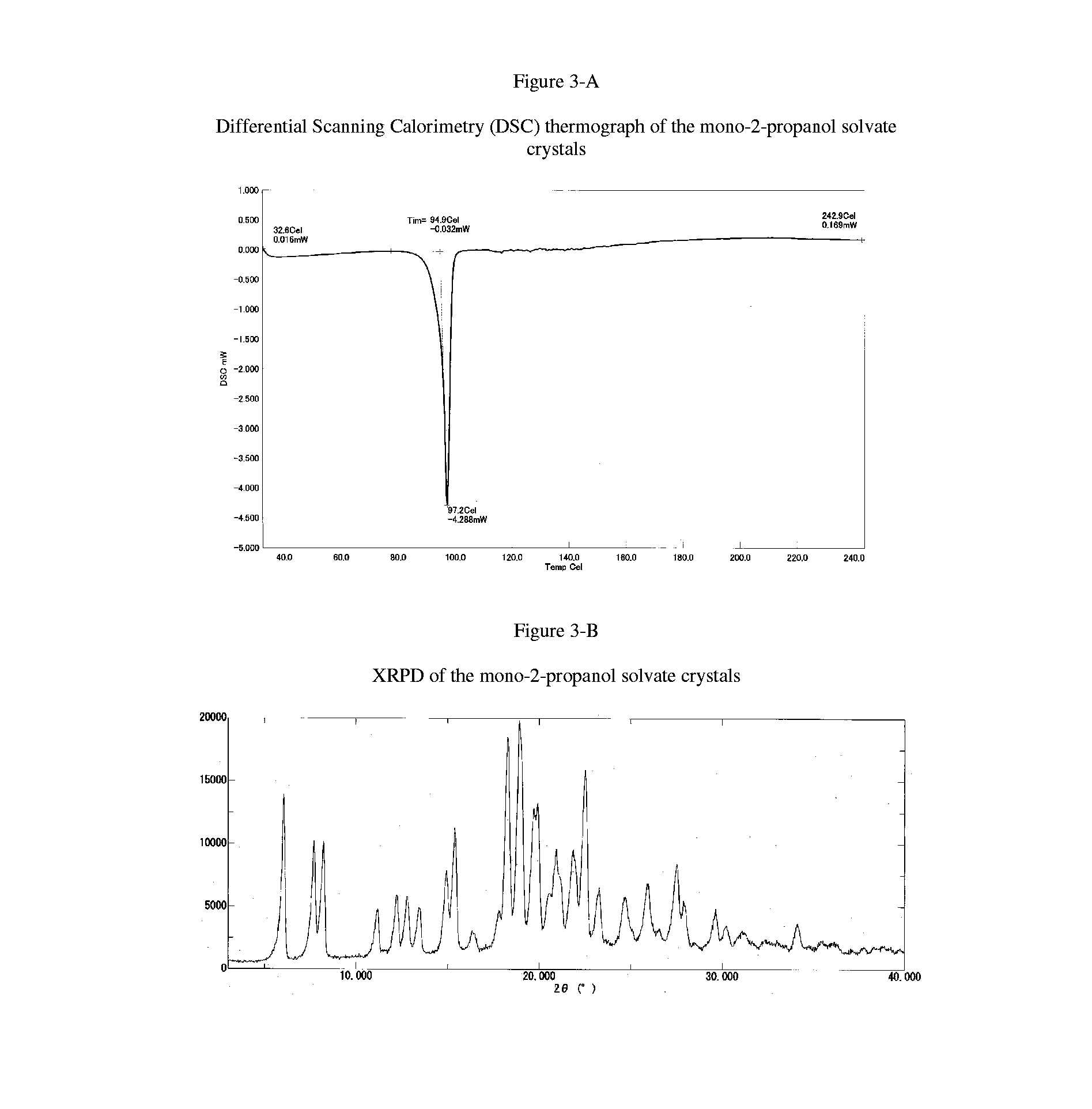
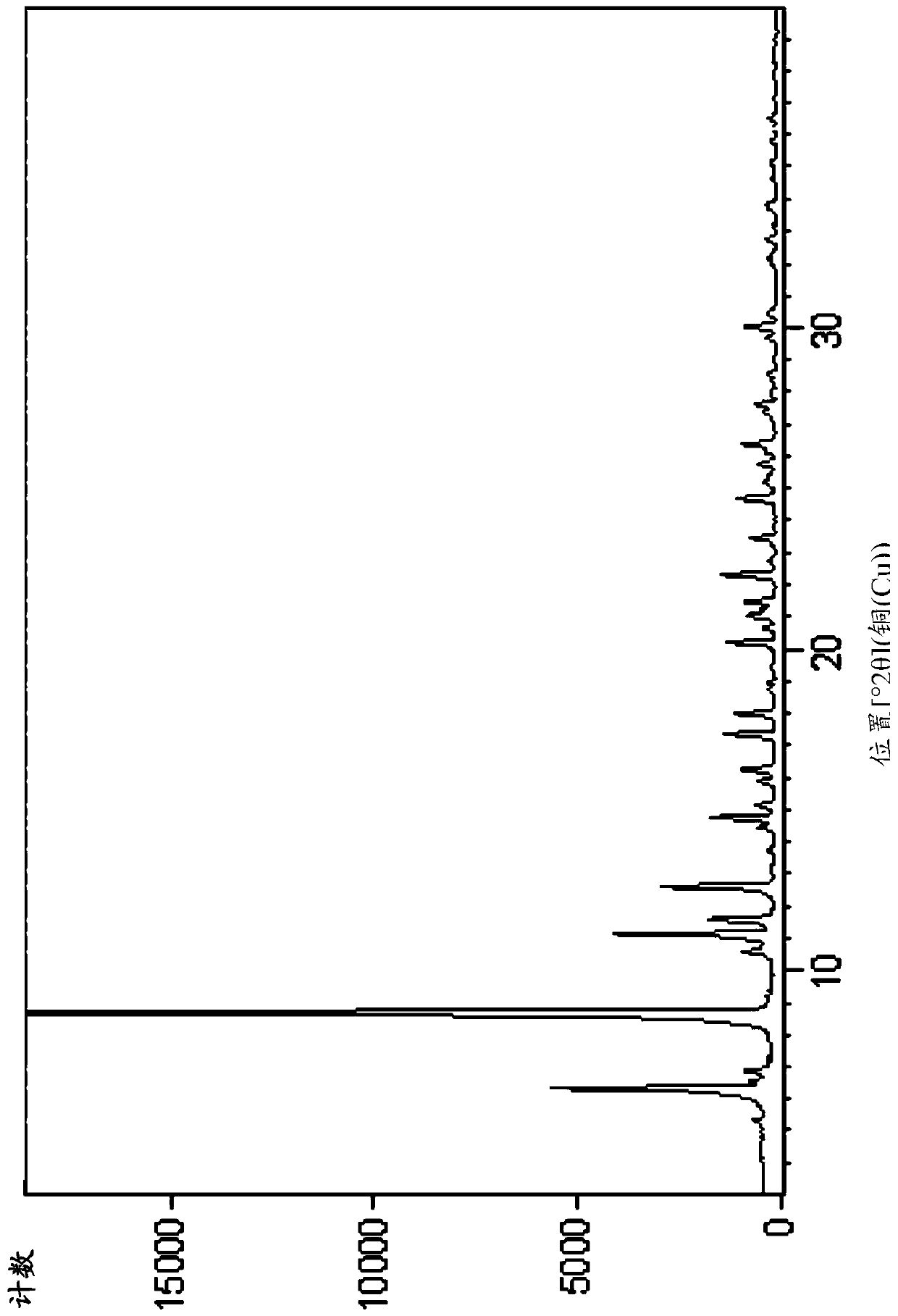
Free base crystals
Owner:INTRA CELLULAR THERAPIES INC
Treatment cancers using combination comprising parp inhibitors, temozolomide and/or radiation therapy
InactiveCN110891576AOrganic active ingredientsOrganic chemistryCancer preventionRadical radiotherapy
Disclosed herein is a method for the prevention, delay of progression or treatment of cancer in a subject, comprising administering to the subject in need thereof a PARP inhibitor, particularly, (R) -2-fluoro-10a-methyl-7, 8, 9, 10, 10a, 11-hexahydro-5, 6, 7a, 11-tetraazacyclohepta [def] cyclopenta [a] fluoren-4 (5H) -one, a sesqui-hydrate thereof, or a pharmaceutically acceptable salt thereof, incombination with temozolomide and / or radiation therapy. Also, disclosed a pharmaceutical combination comprising a PARP inhibitor, particularly, (R) -2-fluoro-10a-methyl-7, 8, 9, 10, 10a, 11-hexahydro-5, 6, 7a, 11-tetraazacyclohepta [def] cyclopenta [a] fluoren-4 (5H) -one, a sesqui-hydrate thereof, or a pharmaceutically acceptable salt thereof, in combination with temozolomide and the use thereof.
Owner:百济神州(苏州)生物科技有限公司
Waterproof eyeliner refill and preparation method thereof
PendingCN112386530AExcellent transfer resistanceExcellent wash-off resistanceCosmetic preparationsMake-upPolymer scienceEngineering
The invention relates to the technical field of eyeliner refills, in particular to a waterproof eyeliner refill prepared from the following raw materials in parts by weight: 10-20 parts of a film-forming agent, 15-18 parts of a thickening agent, 5-10 parts of an emollient, 5-8 parts of a suspending agent, 10-12 parts of a filling agent, 8-15 parts of a coloring agent, 6-10 parts of a skin conditioner, 1-4 parts of a preservative and 2-5 parts of an antioxidant, wherein the film-forming agent comprises one or a compound of more of trimethylsiloxysilicate, cyclopentasiloxane, polypropylsilsesquioxane, a dimethiconol cross-linked polymer and isododecane. By adding the trimethylsiloxysilicate, the cyclopentasiloxane and the isododecane, the product has excellent transfer resistance and elutionresistance, is long-acting and durable, enhances the gloss and color strength of the product, is smooth but not sticky after being dried, and has excellent film-forming property and water resistance.
Owner:英妃(杭州)化妆品有限公司
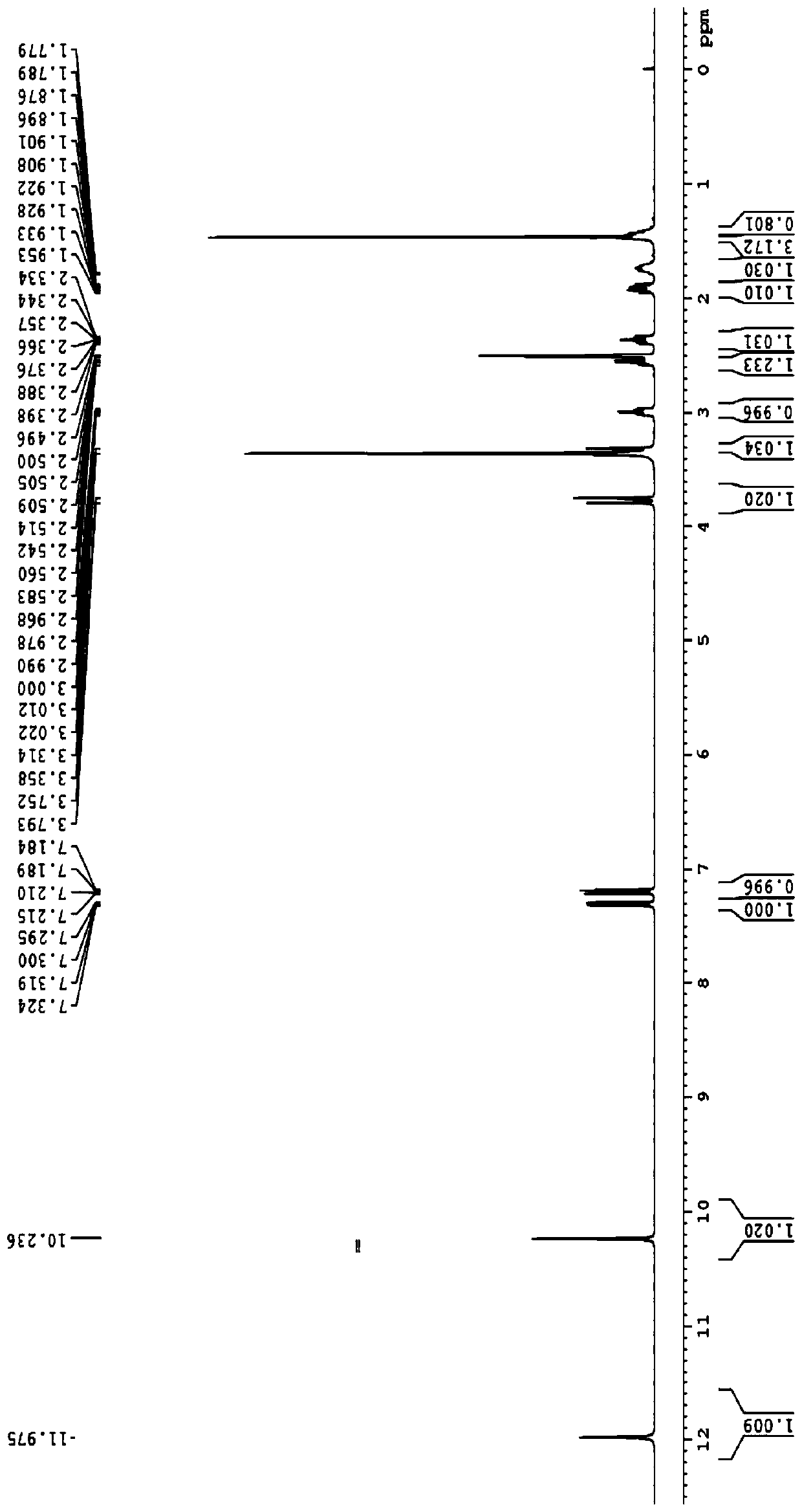
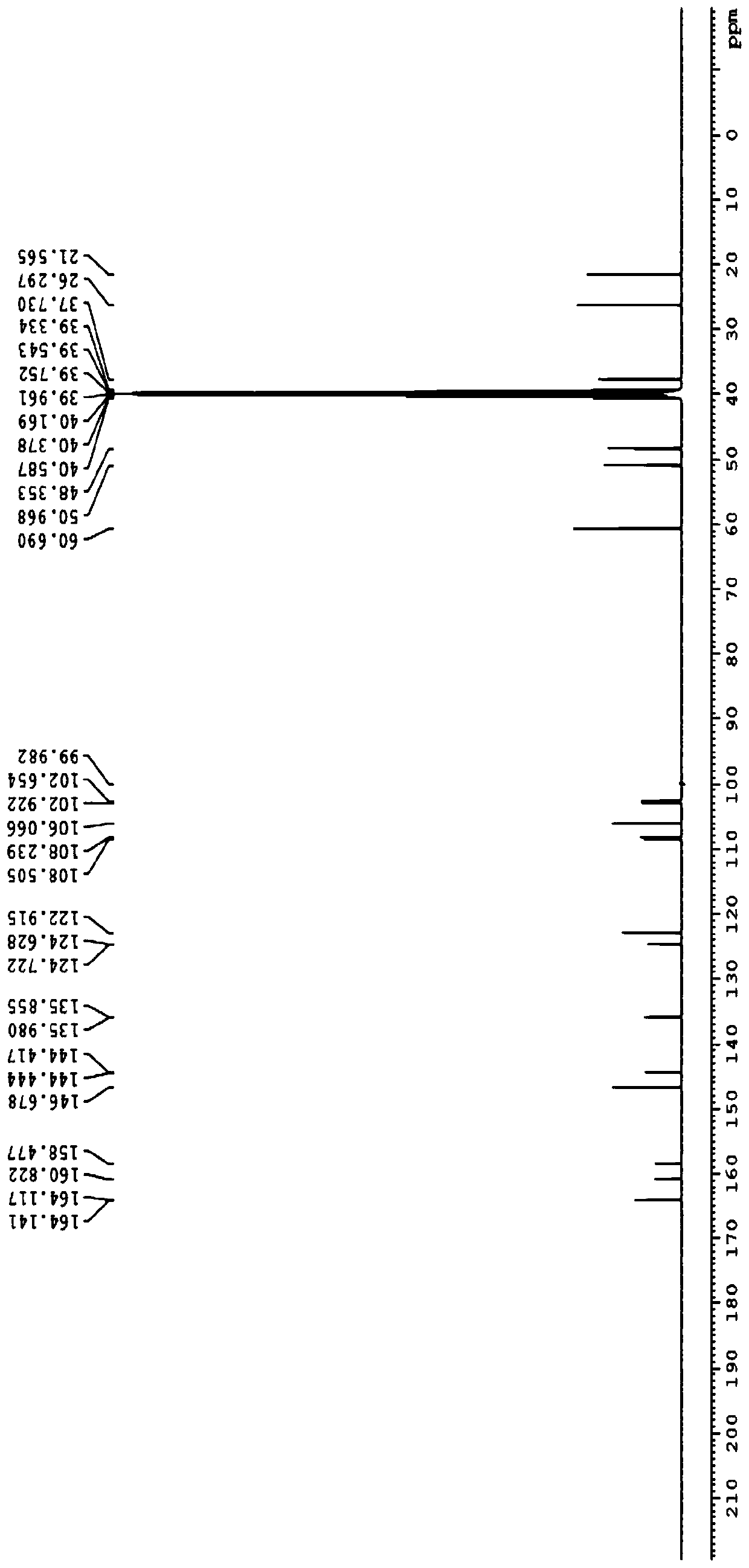
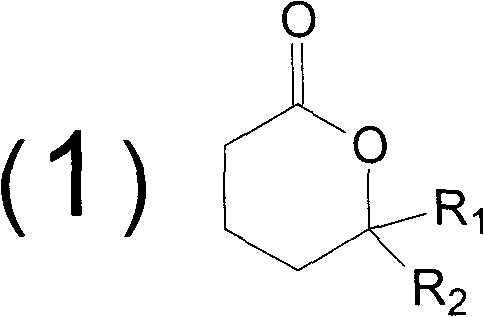
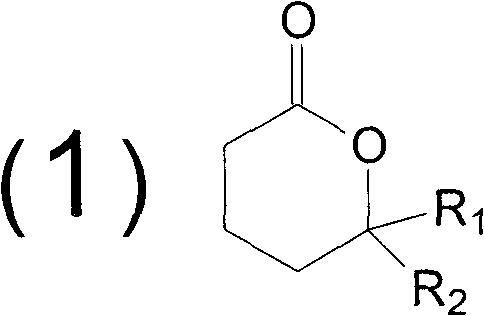
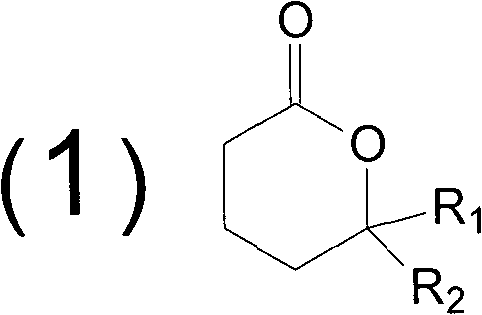
Method for synthesizing butyrolactone and butyrolactone obtained by same
The invention provides a method for synthesizing butyrolactone which is shown as the molecular formula (1) and butyrolactone obtained by the method. The method is characterized by obtaining the butyrolactone by carrying out free radical addition of alcohol and 3-butenoic acid or ester thereof; in the formula (1), R1 is hydrogen, a methyl or an ethyl; and R2 is a propyl, a butyl, an amyl, a hexyl, a heptyl, an octyl, a nonyl, a phenyl, a benzyl, a cyclopentyl, a cyclopentylmethyl, a cyclohexyl or a cyclohexylmethyl. The method can realize clean production and obtain high yield.
Owner:北京北达正元科技有限公司 +1
Shoe polish prepared from wool fat extract and preparation method of shoe polish
InactiveCN113088198AImprove stabilityExtended shelf lifePolishing compositionsMethacrylatePolymer science
The invention discloses shoe polish prepared from a wool fat extract and a preparation method of the shoe polish, and the shoe polish is prepared by taking the modified wool fat extract as a main raw material and simultaneously adding substances such as modified mixed wax, a regulator and an emulsifier. The modified wool fat extract is obtained by modifying a wool fat extract through a modifier, and the modifier is a mixture of sorbitan monooleate, fatty alcohol-polyoxyethylene ether and (1-fluoro-2-phenyl vinyl) (4-aminophenyl) thioether; and the modified mixture is prepared by taking liquid paraffin, microcrystalline wax and carnauba wax as raw materials and under the promotion action of an accelerant, and the accelerant is a mixture of 3, 4, 6-trihydroxy-5-oxo-5H-benzo [7] cyclopent-8-carboxylic acid propyl ester, bisphenol A glycerol dimethacrylate and 1, 2-epoxyhexyl-5-ene. The shoe polish prepared from the lanolin extract enables leather to have excellent glossiness and waterproofness, and meanwhile, the shoe polish has very high wiping resistance and durability and is wide in application range.
Owner:浙江花园营养科技有限公司
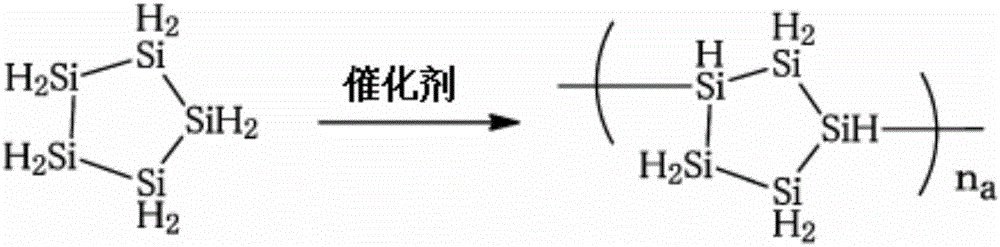
High-molecular-weight polysilane and method for producing same
ActiveCN106660810AResidue reductionProduction cost advantageSiliconSilicon hydridesFunctionalized polystyrenePolymer science
[Problem] To produce an application-type polysilane composition using polysilane having a high weight-average molecular weight, and provide an excellent silicon thin film having high conductivity after applying the polysilane composition to a substrate and firing the substrate. [Solution] Polysilane having a weight-average molecular weight of from 5,000 to 8,000. The polysilane is a polymer of cyclopentasilane. A silicon film obtained by applying a polysilane composition of polysilane dissolved in a solvent to a substrate, and firing at from 100 degrees to 425 degrees. Polymerization of cyclopentasilane is carried out in the presence of a polymer-supported palladium catalyst. In the polymer-supported palladium catalyst, the palladium of the catalyst component is immobilized by functionalized polystyrene. The palladium comprises a palladium compound or a palladium complex. Immobilization of the palladium involves the microencapsulation of a zero-valent palladium complex or divalent palladium compound using functionalized polystyrene. The zero-valent palladium complex comprises a tetrakis(triphenylphosphine)palladium(0) complex.
Owner:THIN FILM ELECTRONICS ASA
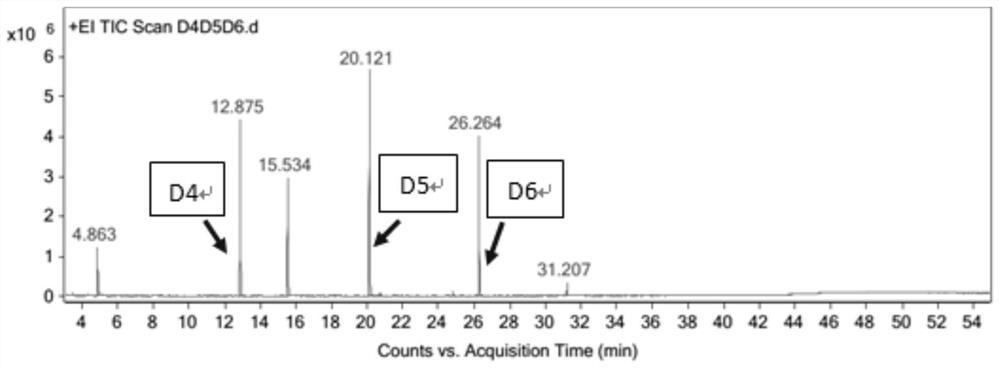
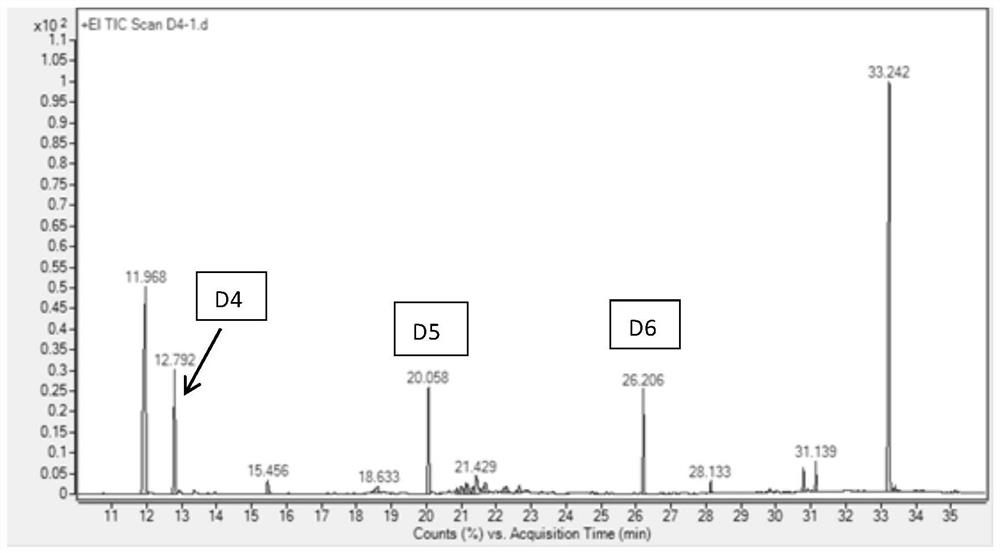
Method for simultaneously determining octamethylcyclotetrasiloxane, decamethylcyclopentasiloxane and dodecamethylcyclohexasiloxane
PendingCN113917063ASimple methodHigh sensitivityComponent separationQuantitative determinationPhysical chemistry
The invention discloses a method for simultaneously determining octamethylcyclotetrasiloxane, decamethylcyclopentasiloxane and dodecamethylcyclohexasiloxane, and belongs to the technical field of component detection. The method comprises the following steps of detecting a to-be-detected sample by adopting a thermal desorption-gas chromatography-mass spectrometry method to obtain a total ion chromatogram and peak areas corresponding to three components, namely the octamethylcyclotetrasiloxane, the decamethylcyclopentasiloxane and the dodecamethylcyclohexasiloxane, and calculating the content of the three components according to a formula shown in description, wherein W is the respective content of the three components, Area is peak areas corresponding to the three components respectively, and msample is the mass of the to-be-detected sample. The method can accurately and effectively carry out qualitative and quantitative determination on octamethylcyclotetrasiloxane, decamethylcyclopentasiloxane and dodecamethylcyclohexasiloxane in the to-be-detected sample, and is simple, high in sensitivity and recovery rate and good in reproducibility.
Owner:JIANGSU MAYSTA CHEM
A polymer containing thienothiophene, benzothiadiazole and cyclopentadithiophene and its preparation and application
ActiveCN103848967BLower bandgapBroaden the range of light absorptionOrganic chemistrySolid-state devicesPolymer scienceCiclopentolato
The present invention provides a polymer containing thienothiophene, benzothiadiazole and cyclopentadithiophene, a preparation method and an application thereof. The polymer is a polymer P having the following general formula, wherein R1 and R2 are the same or different H or C1-C12 alkyl, and n is a natural number of 5-60. According to the present invention, the three units such as thienothiophene, benzothiadiazole and cyclopentadithiophene in the polymer are combined to form the repeated unit having the acceptor-donor-acceptor-donor (A-D-A-D) form so as to enhance the charge transporting in the polymer molecule, reduce the band gap of the polymer, and expand the light absorption range of the polymer material, such that the polymer has characteristics of excellent sunlight matching property, excellent carrier mobility property and simple and controllable preparation method, and has good application prospects in the fields of polymer solar cells, organic electroluminescent devices and other optoelectronic materials. The polymer P is as the follow.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Environment-friendly tracer controlled release capsule and method thereof
InactiveCN112112640AGood sustained release effectImprove stabilityBorehole/well accessoriesCelluloseHydrogen Sulfate
The invention belongs to the field of oilfield tracing, and particularly relates to an environment-friendly tracer controlled release capsule and a method thereof. Aiming at the problems of poor slowrelease and stability of an existing oilfield tracer, the invention provides the following scheme that the controlled release capsule is prepared from the following raw materials in parts by weight: 10 to 15 percent of a biological coloring agent, 10 to 15 percent of an acid-base regulator, 5 to 10 percent of methyl fluorobenzoate, 1 to 5 percent of potassium hydrogen sulfate, 2 to 7 percent of cyclobutylsiloxane, 1 to 5 percent of cyclopentasiloxane, 1 to 5 percent of polyvinyl alcohol, 1 to 5 percent of chitosan, 2 to 6 percent of sodium carboxymethylcellulose, 3 to 7 percent of polyethyleneglycol and 4 to 9 percent of di-iso-decylphthalate. The tracer controlled release capsule provided by the invention is injected into a target stratum along with fluid; when the tracer controlled release capsule is in contact with target fluid, a tracer component starts to be slowly released; and the tracer component is carried out by the target fluid; the concentration of the tracer component issampled and analyzed at a wellhead, so that the aim of monitoring the target fluid is fulfilled.
Owner:权冉(银川)科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Process for the preparation of 1-[cyano(aryl)methyl] cyclohexanol Process for the preparation of 1-[cyano(aryl)methyl] cyclohexanol](https://images-eureka.patsnap.com/patent_img/0eb1978d-3cb7-4186-abac-a4a133c9e665/US20020120164A1-20020829-C00001.png)
![Process for the preparation of 1-[cyano(aryl)methyl] cyclohexanol Process for the preparation of 1-[cyano(aryl)methyl] cyclohexanol](https://images-eureka.patsnap.com/patent_img/0eb1978d-3cb7-4186-abac-a4a133c9e665/US20020120164A1-20020829-C00002.png)
![Process for the preparation of 1-[cyano(aryl)methyl] cyclohexanol Process for the preparation of 1-[cyano(aryl)methyl] cyclohexanol](https://images-eureka.patsnap.com/patent_img/0eb1978d-3cb7-4186-abac-a4a133c9e665/US20020120164A1-20020829-C00003.png)
![Cyclopenta[b]indole derivatives as sPLA2 inhibitors Cyclopenta[b]indole derivatives as sPLA2 inhibitors](https://images-eureka.patsnap.com/patent_img/732c4e03-6e2b-4565-b671-b87ba557b320/US07160909-20070109-Brketclosest.png)
![Cyclopenta[b]indole derivatives as sPLA2 inhibitors Cyclopenta[b]indole derivatives as sPLA2 inhibitors](https://images-eureka.patsnap.com/patent_img/732c4e03-6e2b-4565-b671-b87ba557b320/US07160909-20070109-Brketopenst.png)
![Cyclopenta[b]indole derivatives as sPLA2 inhibitors Cyclopenta[b]indole derivatives as sPLA2 inhibitors](https://images-eureka.patsnap.com/patent_img/732c4e03-6e2b-4565-b671-b87ba557b320/US07160909-20070109-C00001.png)
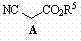

![Process for production of 3-[5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-oxo-2-substituted-2,3-dihydro-1,2-benzisoxazol-6-yl)methoxy]phenyl]propionate ester and intermediate for the process Process for production of 3-[5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-oxo-2-substituted-2,3-dihydro-1,2-benzisoxazol-6-yl)methoxy]phenyl]propionate ester and intermediate for the process](https://images-eureka.patsnap.com/patent_img/21810b87-0394-4ef2-be7b-267a2158566b/US20090099369A1-20090416-C00001.png)
![Process for production of 3-[5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-oxo-2-substituted-2,3-dihydro-1,2-benzisoxazol-6-yl)methoxy]phenyl]propionate ester and intermediate for the process Process for production of 3-[5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-oxo-2-substituted-2,3-dihydro-1,2-benzisoxazol-6-yl)methoxy]phenyl]propionate ester and intermediate for the process](https://images-eureka.patsnap.com/patent_img/21810b87-0394-4ef2-be7b-267a2158566b/US20090099369A1-20090416-C00002.png)
![Process for production of 3-[5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-oxo-2-substituted-2,3-dihydro-1,2-benzisoxazol-6-yl)methoxy]phenyl]propionate ester and intermediate for the process Process for production of 3-[5-[4-(cyclopentyloxy)-2-hydroxybenzoyl]-2-[(3-oxo-2-substituted-2,3-dihydro-1,2-benzisoxazol-6-yl)methoxy]phenyl]propionate ester and intermediate for the process](https://images-eureka.patsnap.com/patent_img/21810b87-0394-4ef2-be7b-267a2158566b/US20090099369A1-20090416-C00003.png)
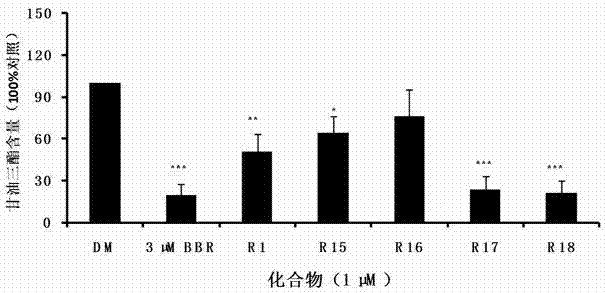
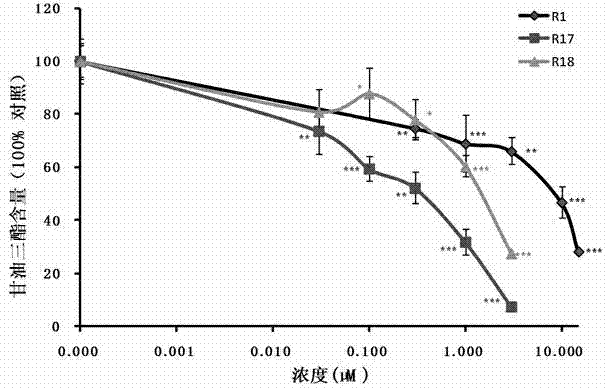
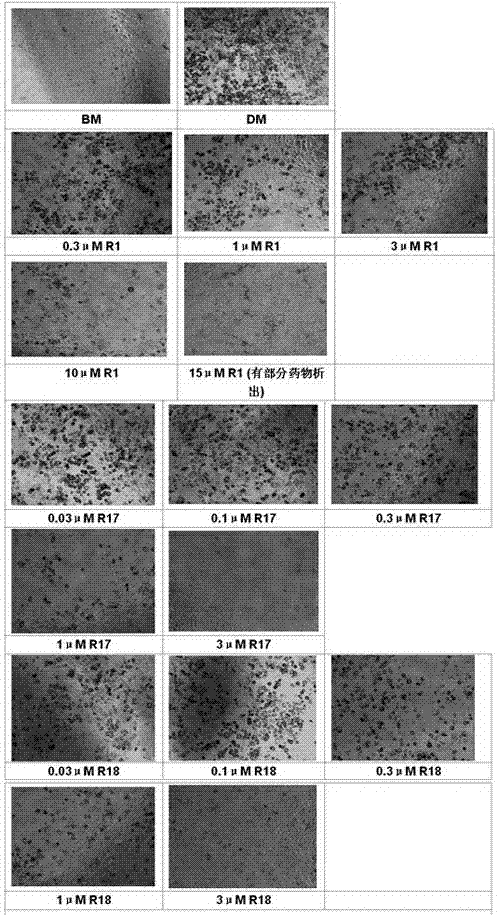
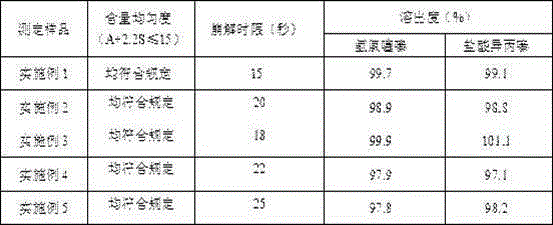
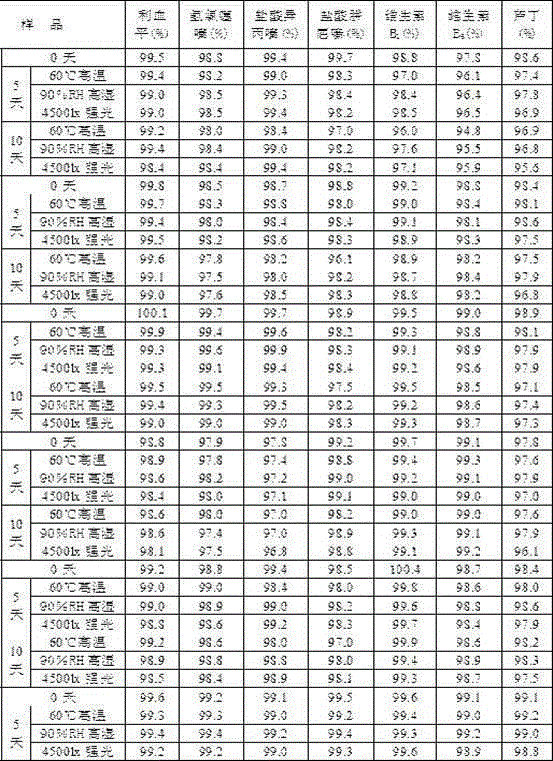
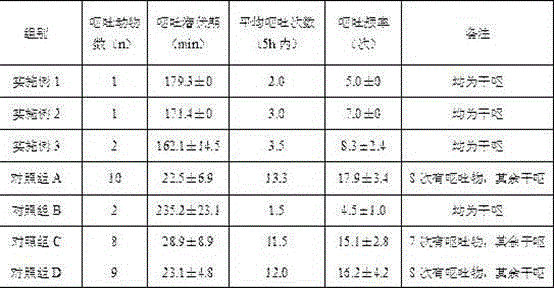
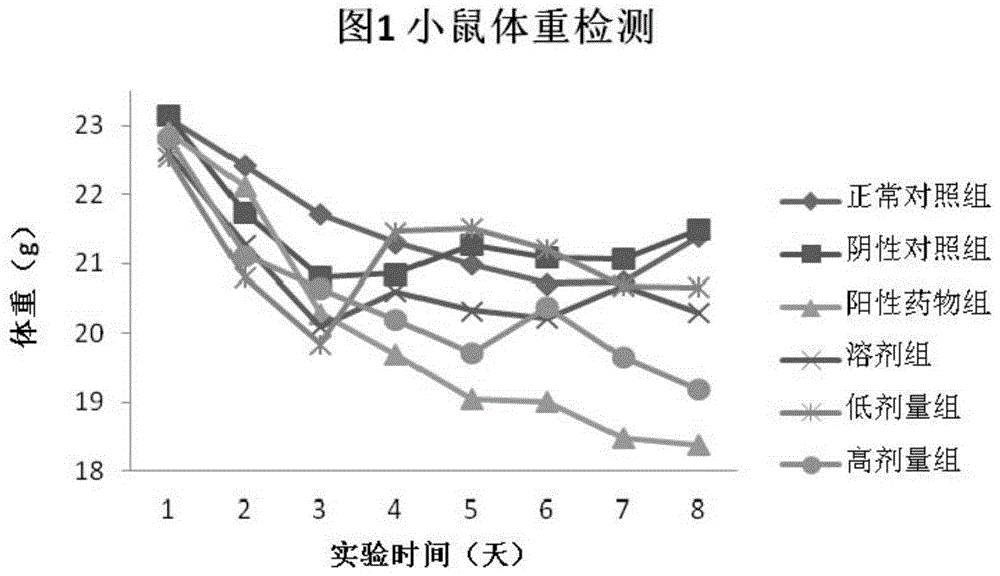
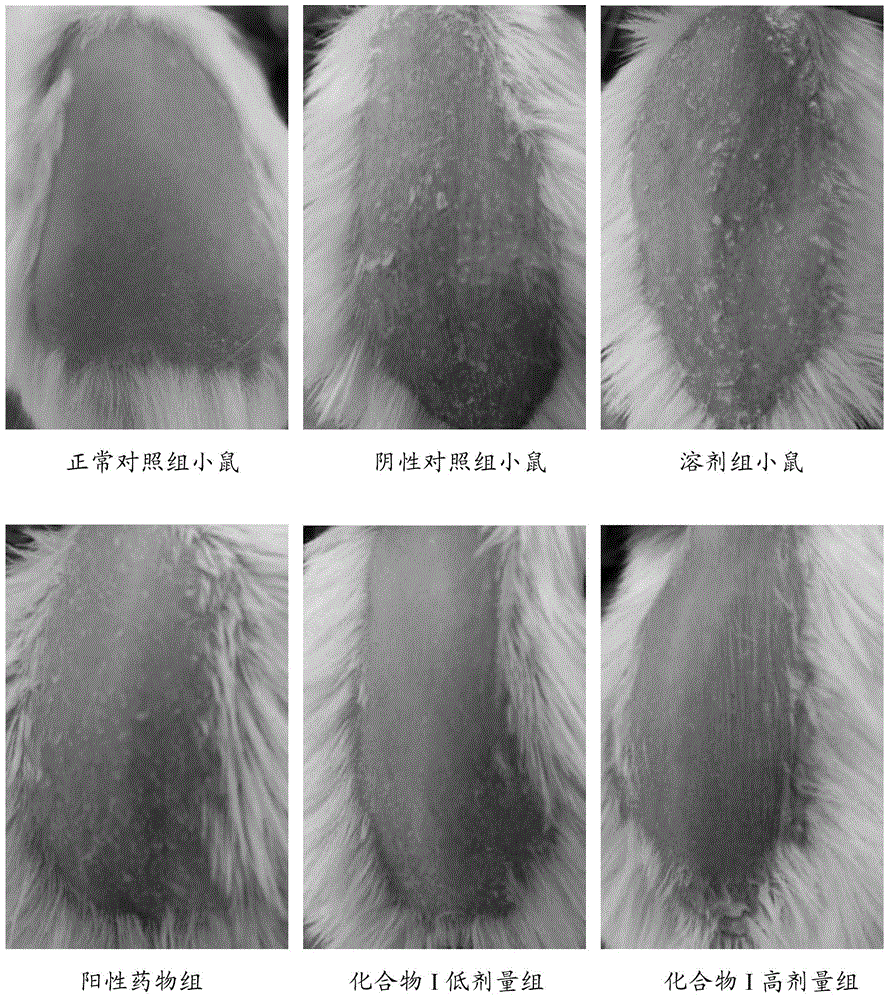
![Preparation method of tetrahydro-cyclopentyl[2,1-b;3,4-b'] bi-thiophene ketone Preparation method of tetrahydro-cyclopentyl[2,1-b;3,4-b'] bi-thiophene ketone](https://images-eureka.patsnap.com/patent_img/05d54387-40a2-4656-8652-dc45876e2f4f/FSA00000097654800011.PNG)
![Preparation method of tetrahydro-cyclopentyl[2,1-b;3,4-b'] bi-thiophene ketone Preparation method of tetrahydro-cyclopentyl[2,1-b;3,4-b'] bi-thiophene ketone](https://images-eureka.patsnap.com/patent_img/05d54387-40a2-4656-8652-dc45876e2f4f/GSA00000097654900011.PNG)
![Preparation method of tetrahydro-cyclopentyl[2,1-b;3,4-b'] bi-thiophene ketone Preparation method of tetrahydro-cyclopentyl[2,1-b;3,4-b'] bi-thiophene ketone](https://images-eureka.patsnap.com/patent_img/05d54387-40a2-4656-8652-dc45876e2f4f/GSA00000097654900012.PNG)
![Cyclopenta [d] pyrimidine compound, pharmaceutically acceptable salt, solvate or prodrug thereof and application Cyclopenta [d] pyrimidine compound, pharmaceutically acceptable salt, solvate or prodrug thereof and application](https://images-eureka.patsnap.com/patent_img/4d88f50f-d31e-4f6a-b4a9-0707cc277d7a/HDA0002411984520000011.png)
![Cyclopenta [d] pyrimidine compound, pharmaceutically acceptable salt, solvate or prodrug thereof and application Cyclopenta [d] pyrimidine compound, pharmaceutically acceptable salt, solvate or prodrug thereof and application](https://images-eureka.patsnap.com/patent_img/4d88f50f-d31e-4f6a-b4a9-0707cc277d7a/HDA0002411984520000012.png)
![Cyclopenta [d] pyrimidine compound, pharmaceutically acceptable salt, solvate or prodrug thereof and application Cyclopenta [d] pyrimidine compound, pharmaceutically acceptable salt, solvate or prodrug thereof and application](https://images-eureka.patsnap.com/patent_img/4d88f50f-d31e-4f6a-b4a9-0707cc277d7a/HDA0002411984520000021.png)