Compound reserpine orally disintegrating tablet for treating hypertension and preparation method of compound reserpine orally disintegrating tablet
A technology of orally disintegrating tablets and compound reserpine, which is applied in the field of pharmaceuticals, can solve the problems of drug taste, long onset time, and large blood pressure fluctuations, and achieve the goals of ensuring quality and efficacy, reducing toxic and side effects, and compliance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
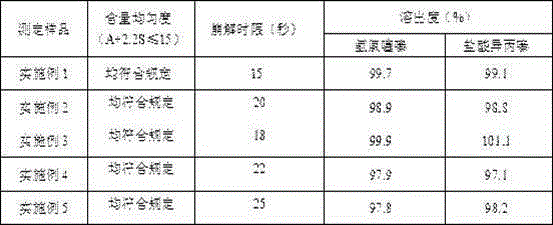
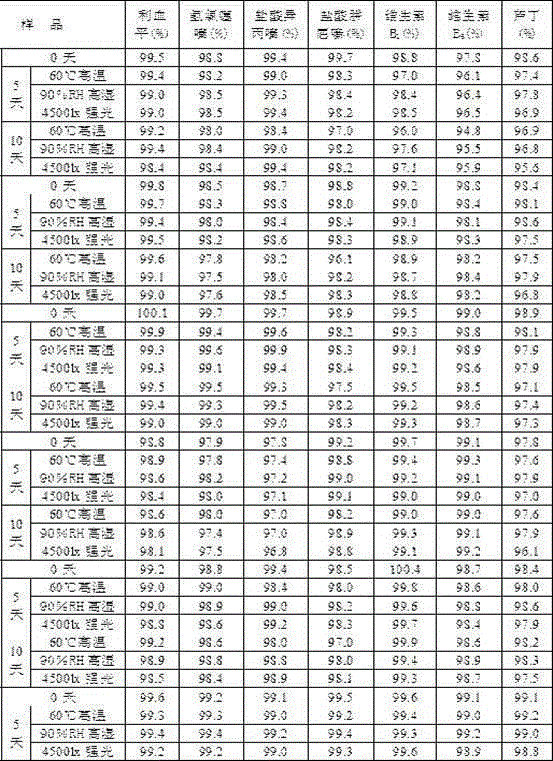
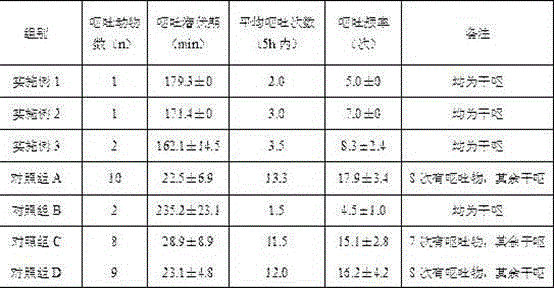
Embodiment 1
[0042] Embodiment 1 compound reserpine tablet and preparation method thereof
[0043] Dissolve the prescribed amount of reserpine, pentathiazine and hydroxypropyl cellulose in ethanol first, add the other auxiliary materials except the lubricant into the fluidized bed granulation coating machine, and spray them into the above-mentioned After the ethanol solution is granulated, it is dried, after the granulation is sized, a lubricant is added, mixed evenly, and tabletted.
[0044] (1) Orally disintegrating tablet prescription
[0045] Reserpine 0.3g, hydralazine hydrochloride 10g, pentathiazide 0.25g, hydrochlorothiazide 15g, promethazine hydrochloride 20g, potassium chloride 300g, rutin 50g, chloroquine phosphate 25g, vitamin B110g, vitamin B610g, fumaric acid 50g, polyacrylic resin IV 200g, mannitol 913g, croscarmellose sodium 36g, hydroxypropyl cellulose 50g, aspartame 50g and magnesium stearate 10g.
[0046] (2) Preparation process
[0047] Weigh the prescribed amount of...
Embodiment 2
[0051] Embodiment 2 compound reserpine tablet and preparation method thereof
[0052] Basically the same as Example 1, the difference is:
[0053] The prescription of compound reserpine tablet of the present invention consists of:
[0054] Reserpine 0.6g, Hydralazine Hydrochloride 20g, Cyclopenthiazine 0.5g, Hydrochlorothiazide 30g, Promethazine Hydrochloride 40g, Potassium Chloride 600g, Rutin 100g, Chloroquine Phosphate 50g, Vitamin B120g, Vitamin B620g, Fumaric Acid 120g, polyacrylic resin IV500g, mannitol 913g, microcrystalline cellulose 913g, croscarmellose sodium 70g, hydroxypropyl cellulose 100g, aspartame 80g and sodium stearate fumarate 30g.
[0055] Explanation: The purified water and ethanol added in this example are prepared through the preparation method and finally dried to obtain the product, and the added purified water and ethanol are all evaporated;
[0056] The preparation process of the compound reserpine tablet of the present invention is the same as that ...
Embodiment 3
[0057] Embodiment 3 compound reserpine tablet and preparation method thereof
[0058] Basically the same as Example 1, the difference is:
[0059] The prescription of compound reserpine tablet of the present invention consists of:
[0060] Reserpine 0.3g, hydralazine hydrochloride 10g, pentathiazide 0.25g, hydrochlorothiazide 15g, promethazine hydrochloride 20g, potassium chloride 300g, rutin 50g, chloroquine phosphate 25g, vitamin B110g, vitamin B610g, fumaric acid 70g, polyacrylic acid resin IV300g, mannitol 1000g, croscarmellose sodium 35g, hydroxypropyl cellulose 40g, aspartame 50g, citric acid 50g and magnesium stearate 10g.
[0061] Explanation: The purified water and ethanol added in this example are prepared through the preparation method and finally dried to obtain the product, and the added purified water and ethanol are all evaporated;
[0062] The preparation process of the compound reserpine tablet of the present invention is the same as that in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com